Animal Healthcare Hygiene & Disinfection Chemicals
What is animal hygiene?
Animal Hygiene is the branch of veterinary science, which relies on knowledge of physiological and ethological demands of animals, describes the preconditions of health preservation and investigates the pathophysiological changes brought about by adverse environmental effects to gain information on the aetiology and pathomechanism of multifactorial diseases.
Why is animal health important?
Monitoring animal health and preventing animal disease outbreaks is vital to the economy and safety of the country’s food supply. The production of healthy livestock helps to ensure a safe food supply and keep consumer prices stable.
Automatic Vehicles Disinfection
Farm Animal Disinfection
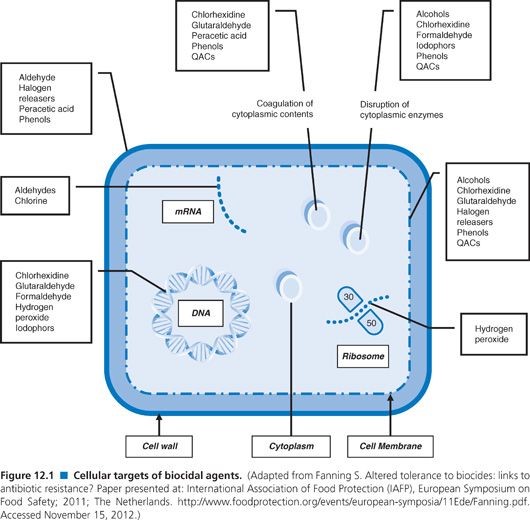
Formaldehyde
Primarily available as a water-based solution called formalin, which contains 37% formaldehyde by weight – is used as a high-level disinfectant and sterilant. Formaldehyde exerts its bactericidal, tuberculocidal, fungicidal, virucidal and sporicidal effects in the aqueous state, as well as in combination with low-temperature steam.
This extremely reactive chemical’s mechanism of action is attributed to its interactive and cross-linking properties with protein, DNA and RNA in vitro, resulting in the disruption of DNA synthesis. It can also penetrate bacterial spores.
Formaldehyde has been traditionally used to sterilize equipment such as surgical instruments and haemodialysers in combination with alcohols. Paraformaldehyde, a solid polymer of formaldehyde, is used in combination with low-temperature steam for the disinfection of heat-sensitive medical equipment. Exposure hazards and the potential carcinogenic effects of formaldehyde have limited its healthcare uses recently, while next-generation aldehydes such as glutaraldehyde and o-phthalaldehyde, with better sporicidal activity, faster onset of action and lesser exposure effects, have replaced formaldehyde in the disinfection manuals of most healthcare establishments.
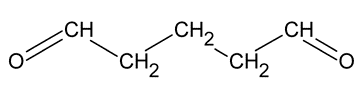
Glutaraldehyde
Glutaraldehyde is a saturated dialdehyde widely used as a potent sterilant and high-level disinfectant. Its broad-spectrum, covering bactericidal, sporicidal, fungicidal and virucidal activity, makes it an ideal chemical for the low-temperature disinfection and sterilisation of critical and semi-critical equipment such as endoscopes, dialysers and surgical tools.
“Glutaraldehyde exposure should be monitored to ensure a safe working environment.”
Aqueous solutions of glutaraldehyde when activated (rendered alkaline at 7.5- 8.5 pH) become sporicidal, while its microbicidal action is attributed primarily to its strong association with the outer layers of bacterial cells and the alkylation of sulphydryl, hydroxyl, carboxyl and amino groups, which alter RNA, DNA and protein synthesis within microorganisms.
Glutaraldehyde is a saturated dialdehyde that has gained wide acceptance as a high-level disinfectant and chemical sterilant. Aqueous solutions of glutaraldehyde are acidic and generally in this state are not sporicidal. Only when the solution is “activated” (made alkaline) by the use of alkalinising agents to pH 7.5–8.5 does the solution become sporicidal. Once activated, these solutions have a shelf-life of minimally 14 days because of the polymerization of the glutaraldehyde molecules at alkaline pH levels. This polymerization blocks the active sites (aldehyde groups) of the glutaraldehyde molecules that are responsible for its biocidal activity.
Glutaraldehyde is also mycobactericidal, and glutaraldehyde formulations with phenols and alcohols have better usage life while maintaining excellent microbicidal activity. Glutaraldehyde exposure should be monitored to ensure a safe working environment. Acute or chronic exposure, resulting in skin irritation, mucous membrane irritation and multiple pulmonary symptoms such as occupational asthma and allergic rhinitis have been reported in healthcare workers.
Due to its exposure hazards, US healthcare associations advocate the use of glutaraldehyde alternatives such as o-phthalaldehyde, hydrogen peroxide and peracetic acid.
Ortho-phthalaldehyde
Since its introduction in 1999, ortho-phthalaldehyde (OPA) has been accepted as a better, safer alternative to glutaraldehyde in most US healthcare facilities. OPA by Advanced Sterilization Products was cleared by the US FDA as a high-level disinfectant and emerged as a suitable replacement of glutaraldehyde for the disinfection of endoscopes.
OPA has excellent microbicidal activity and superior mycobactericidal activity compared with glutaraldehyde and has potent bactericidal and sporicidal activity. Like glutaraldehyde, it interacts with amino acids, proteins and microorganisms.
OPA has many advantages over glutaraldehyde, such as improved stability at varying pH ranges, lower inhalation exposure risk and a wider range of material compatibility, although it costs almost three times more; but, considering the cost of the sophisticated ventilation systems needed to minimize the respiratory hazards of using glutaraldehyde, OPA is more economical.
Ortho-phthalaldehyde is a high-level disinfectant that received FDA clearance in October 1999. It contains 0.55% 1,2-benzenedicarboxaldehyde (OPA). OPA solution is a clear, pale-blue liquid with a pH of 7.5.
Hydrogen peroxide
Disinfectant solutions containing 7.5% hydrogen peroxide have been approved by the US FDA for sterilization and high-level disinfection in healthcare settings.
“Disinfectant solutions containing 7.5% hydrogen peroxide have been approved by the US FDA for sterilization.“ A 0.5% accelerated hydrogen peroxide demonstrated bactericidal and virucidal activity in 1 minute and mycobactericidal and fungicidal activity in 5 minutes
Its good, broad-spectrum bactericidal, virucidal, sporicidal and fungicidal properties, combined with its excellent stability and environmentally friendly characteristics, have made hydrogen peroxide the disinfectant of choice for semi-critical and non-critical equipment while being an ideal surface disinfectant.
Despite not being listed as equipment-compatible by all endoscope manufacturers, hydrogen peroxide-based disinfectant solutions are still considered ideal alternatives for other toxic sterilants, such as ethylene oxide and aldehyde disinfectants. Hydrogen peroxide produces destructive hydroxyl-free radicals that act on membrane lipids, DNA and other essential cell components.
Peracetic Acid
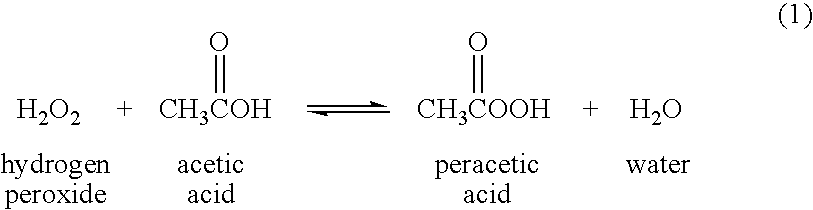
Peracetic, or peroxyacetic, acid is characterized by rapid action against all microorganisms. Special advantages of peracetic acid are that it lacks harmful decomposition products (i.e., acetic acid, water, oxygen, hydrogen peroxide), enhances the removal of organic material, and leaves no residue. It remains effective in the presence of organic matter and is sporicidal even at low temperatures. Peracetic acid can corrode copper, brass, bronze, plain steel, and galvanized iron but these effects can be reduced by additives and pH modifications. It is considered unstable, particularly when diluted; for example, a 1% solution loses half its strength through hydrolysis in 6 days, whereas 40% peracetic acid loses 1%–2% of its active ingredients per month
Another emerging alternative to ethylene oxide and aldehyde sterilants is peracetic acid. Peracetic acid-based solutions are considered to be a more potent disinfectant than hydrogen peroxide; are sporicidal, bactericidal, virucidal and fungicidal at low concentrations; and are environment friendly. They have replaced traditional disinfectants for medical devices, endoscopes and haemodialysers.
It also acts as an environmental surface sterilant, and behaves similarly to other oxidising agents, disrupting cell wall permeability and oxidising sulphhydryl and sulphur bonds in proteins, enzymes and other metabolites.
Hydrogen Peroxide and Peracetic Acid Combination
Two chemical sterilants are available that contain peracetic acid plus hydrogen peroxide (i.e., 0.08% peracetic acid plus 1.0% hydrogen peroxide [no longer marketed]; and 0.23% peracetic acid plus 7.35% hydrogen peroxide

The 0.08% peracetic acid plus 1.0% hydrogen peroxide product effectively inactivated glutaraldehyde-resistant mycobacteria
Peracetic acid, when combined with hydrogen peroxide, was found to be more effective, typically against glutaraldehyde-resistant mycobacteria. Combination of products cleared by the FDA.
The combination of peracetic acid and hydrogen peroxide has been used for disinfecting haemodialysers, but the mix was found to be incompatible with flexible gastrointestinal endoscopes as claimed by leading US manufacturers.
Intermediate-level Disinfectants
Sodium hypochlorite
Chlorine-releasing agents (CRAs), the most popular sodium hypochlorite solution, are widely used for the disinfection of hard surfaces and blood spillages containing the human immunodeficiency virus or hepatitis B virus.


Recently, sodium hypochlorite was designated as the best defence against hospital-acquired and community-acquired Clostridium difficile infections. With a broad spectrum of antimicrobial activity, sodium hypochlorite is inexpensive with low toxicity.
CRAs are highly active oxidising agents and destroy the cellular activity of proteins. Hypochlorous acid, the active moiety, has been shown to have harmful effects on bacterial DNA through the formation of chlorinated derivatives of nucleotide bases.
High concentrations of sodium hypochlorite display significant levels of sporicidal and virucidal activity. Sodium hydroxide (7681-52-9) – 15-30%. Sodium hypochlorite (1310-73-2)-3-5%

The most prevalent chlorine products in the United States are aqueous solutions of 5.25%–6.15% sodium hypochlorite (see glossary), usually called household bleach.
They have a broad spectrum of antimicrobial activity, do not leave toxic residues, are unaffected by water hardness, are inexpensive and fast-acting, remove dried or fixed organisms and biofilms from surfaces and have a low incidence of serious toxicity
After reviewing environmental fate and ecologic data, EPA has determined the currently registered uses of hypochlorites will not result in unreasonable adverse effects on the environment


Iodophors
Iodophors, complexes of iodine and a solubilising agent or carrier, are used as antiseptics and surface disinfectants. Iodine has bactericidal, fungicidal, tuberculocidal, virucidal and sporicidal properties, while its antiseptic properties are well known.
Iodophors, such as povidone-iodine and poloxamer-iodine, are much more stable, have fewer irritant characteristics and exert better microbicidal action than aqueous iodine solutions. The FDA has not cleared any liquid chemical sterilant or high-level disinfectant with iodophors as the main active ingredient, but iodophors are still used in healthcare settings for disinfecting blood culture bottles and medical equipment such as thermometers.

Low-level Disinfectants
Phenols
“Phenolic disinfectants disrupt the cell membrane of microorganisms.”
Since Dr Joseph Lister’s use of phenols for his pioneering work on antiseptic surgery, phenolic disinfectants have been used as low and intermediate-level disinfectants. Phenolic disinfectants are effective bactericides, fungicides, tuberculocides and virucides, but are ineffective against spore-forming bacteria such as Clostridium difficile.


EPA-registered phenolic disinfectants are used to disinfect surface areas and non-critical medical devices. Phenolics are not FDA-cleared as high-level disinfectants for use with semi-critical items.

Phenolic disinfectants disrupt the cell membrane of microorganisms, and two phenol derivatives used commonly in hospital disinfectants are orthophenylphenol and ortho-benzyl-parachlorophenol.
Quaternary ammonium compounds
Quaternary ammonium compounds (QACs) are used for a variety of clinical purposes such as preoperative disinfection, disinfection of non-critical instruments, and hard-surface cleaning and deodorisation.

QACs possess bactericidal, fungicidal and virucidal properties; however, they only display mycobacteria static and spore static activity. Quaternary ammonium disinfectants contain NH4+, the strong positive charge that results in better contact with negatively charged surfaces, making them good cleaning agents.
EPA-registered QACs are appropriate for use in disinfecting medical equipment that contacts unbroken skin, such as blood pressure cuffs. US hospitals also use quaternary ammonium-based disinfectants for the terminal disinfection of hospital rooms.
Chemically, the quaternary is organically substituted ammonium compounds in which the nitrogen atom has a valence of 5, four of the substituent radicals (R1-R4) are alkyl or heterocyclic radicals of a given size or chain length, and the fifth (X‑) is a halide, sulfate, or similar radical. Each compound exhibits its antimicrobial characteristics, hence the search for one compound with outstanding antimicrobial properties. Some of the chemical names of quaternary ammonium compounds used in healthcare are alkyl dimethyl benzyl ammonium chloride, Alkyl didecyl dimethyl ammonium chloride, and dialkyl dimethyl ammonium chloride. The newer quaternary ammonium compounds (i.e., fourth-generation), referred to as twin-chain or dialkyl quaternary (e.g.) Didecyl dimethyl ammonium bromide and dioctyl dimethyl ammonium bromide

Purportedly remain active in hard water and are tolerant of anionic residues.
A few case reports have documented occupational asthma as a result of exposure to benzalkonium chloride

Table: 1
Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants
| Chemical Characteristics | Hydrogen Peroxide (7.5%) | Peracetic Acid (0.2%) | Glutaraldehyde (≥2.0%) | OPA (0.55%) | Hydrogen Peroxide / Peracetic Acid (7.35%/0.23%) |
| High-level disinfectant claim | 30 minutes @ 20°C | Not Applicable | 20-90 minutes @ 20o-25°C | 12 minutes @ 20°C, 5 minutes @ 25°C in AER | 15 minutes @ 20°C |
| Sterilization Claim | 6 hours @ 20°C | 12 minutes @ 50-56°C | 10 hours @ 20o-25°C | None | 3 h @ 20°C |
| Activation | No | No | Yes (alkaline glutaraldehyde) | No | No |
| Reuse life (number of days a product can be reused as determined by re-use protocol) | 21 days | Single-use | 14-30 days | 14 days | 14 days |
| Shelf-life stability (the time a product can remain in storage (unused)) | 2 years | 6 months | 2 years | 2 years | 2 years |
| Disposal Restrictions | None | None | Local (no U.S. EPA regulations exist but some states and local authorities have disposal restrictions) | Local (no U.S. EPA regulations exist but some states and local authorities have disposal restrictions) | None |
| Materials Compatibility | Good | Good | Excellent | Excellent | No data |
| Monitor MEC of solution | Yes (6%) | No | Yes (1.5% or higher) | Yes (0.3% OPA) | No |
| Safety | Serious eye irritant (safety glasses) | Serious eye and skin irritant (conc. Soln.) 5 | Respiratory irritant | Eye irritant, stains skin | Eye irritant |
| Processing | Manual or automated | Automated | Manual or automated | Manual or automated | Manual |
| Organic material resistance | Yes | Yes | Yes | Yes | Yes |
| OSHA exposure limit | 1 ppm TWA | None | None (The ceiling limit recommended by the American Conference of Governmental Industrial Hygienists is 0.05 ppm.) | None | Hydrogen Peroxide -1 ppm (time-weighted average for a conventional 8-hour workday) |
| Cost profile (per cycle)1 | + (manual) ++ (automated) | +++++ (automated) | + (manual) ++ (automated) | ++ (manual) | ++ (manual) |
Abbreviations and Footnotes:
OPA ortho-phthalaldehyde (FDA cleared as a high-level disinfectant, included for comparison to other chemical agents used for high-level disinfection)
AER Automated Endoscope Reprocessor
MEC minimum effective concentration is the lowest concentration of active ingredients at which the product is still effective
1 per cycle cost profile considers the cost of the processing solution (suggested list price to healthcare facilities in August 2001) and assumes maximum use life (e.g., 21 days for hydrogen peroxide, 14 days for glutaraldehyde), 5 reprocessing cycles per day, 1-gallon basin for manual processing, and 4-gallon tank for automated processing.
+ = least expensive;
+++++ = most expensive
Table: 2
Summary of advantages and disadvantages of chemical agents used as chemical sterilants1 or as high-level disinfectants
| Sterilization Method | Advantages | Disadvantages |
|---|---|---|
| Peracetic Acid/Hydrogen Peroxide | · No activation required · Odour or irritation not significant | · Materials compatibility concerns (lead, brass, copper, zinc) both cosmetic and functional · Limited clinical experience · Potential for eye and skin damage |
| Glutaraldehyde | · Numerous use studies published · Relatively inexpensive · Excellent materials compatibility | · Respiratory irritation from glutaraldehyde vapour · Pungent and irritating odour · Relatively slow mycobactericidal activity · Coagulates blood and fixes tissue to surfaces · Allergic contact dermatitis · Glutaraldehyde vapour monitoring recommended |
| Hydrogen Peroxide | · No activation required · May enhance the removal of organic matter and organisms · No disposal issues · No odour or irritation issues · Does not coagulate blood or fix tissues to surfaces · Inactivates Cryptosporidium · Use studies published | · Material compatibility concerns (brass, zinc, copper, and nickel/silver plating) both cosmetic and functional · Serious eye damage with contact |
| Ortho-phthalaldehyde | · Fast-acting high-level disinfectant · No activation required · Odour not significant · Excellent materials compatibility claimed · Does not coagulate blood or fix tissues to surfaces claimed | · Stains skin, mucous membranes, clothing, and environmental surfaces · Repeated exposure may result in hypersensitivity in some patients with bladder cancer · More expensive than glutaraldehyde · Eye irritation with contact · Slow sporicidal activity |
| Peracetic Acid | · Rapid sterilization cycle time (30-45 minutes) · Low temperature (50-55°C) liquid immersion sterilization · Environmentally friendly by-products (acetic acid, O2, H20) · Fully automated · The single-use system eliminates the need for concentration testing · Standardized cycle · May enhance the removal of organic material and endotoxin · No adverse health effects on operators under normal operating conditions · Compatible with many materials and instruments · Does not coagulate blood or fix tissues to surfaces · Sterilant flows through scope facilitating salt, protein, and microbe removal · Rapidly sporicidal · Provides procedure standardization (constant dilution, perfusion of the channel, temperatures, exposure) | · Potential material incompatibility (e.g., the aluminium anodized coating becomes dull) · Used for immiscible instruments only · The biological indicator may not be suitable for routine monitoring · One scope or a small number of instruments can be processed in a cycle · More expensive (endoscope repairs, operating costs, purchase costs) than high-level disinfection · Serious eye and skin damage (concentrated solution) with contact · The point-of-use system, no sterile storage |
Modified from Rutala.
1 All products effective in presence of organic soil, relatively easy to use, and have a broad spectrum of antimicrobial activity (bacteria, fungi, viruses, bacterial spores, and mycobacteria). The above characteristics are documented in the literature; contact the manufacturer of the instrument and sterilant for additional information. All products listed above are FDA-cleared as chemical sterilants except OPA, which is an FDA-cleared high-level disinfectant.
Probiotics, Prebiotic & Symbiotic products for Animal Performances
We’d love to hear your feedback or answer any questions you may have.
Probiotic Use Growing in Animal Feed and Nutrition
High probiotic demand from farmers and the companion pet sectors are expected to boost the adoption of advanced probiotic technologies for animals, according to Grand View Research.
Probiotic use in animal nutrition is widely accepted today. Rapid advancements in molecular biology and gene sequencing are helping researchers dig deep on finding new probiotic applications for animals, including in feed and consumer-packaged health products for companion pets.
Probiotics are increasingly being added to commercial animal feed for cattle and poultry to alter the gastrointestinal flora and overall animal. Growing awareness among pet owners, cattle farmers, and hobbyists regarding the advantages of probiotics for animal’s health is a major opportunity for the businesses operating in probiotics manufacturing
Livestock Nutrition
Over the last decade or so, there has been a significant rise in the adoption of Probiotics in animal feed for farm animals like swine, cattle, horses, ruminants, and poultry. Using probiotics in cattle feed has shown beneficial results in terms of animal performance, digestion, and the immune system.
When introduced to feed in an appropriate quantity, live probiotic microorganisms can produce bountiful health benefits. For instance, ruminants see numerous benefits when probiotics are included in feed during weaning and at the beginning of lactation.
Another example: yeast helps stabilize pH and reduce the risk of acidosis among ruminants. Today, yeast is commonly used among swine, poultry, and monogastric animals. Yeast-based probiotics target the animal’s colon, caecum, and other areas-a hideouts for diverse microbial populations.
The shift towards antibiotic-free meat is also expected to be a major factor for driving businesses to discover safe and reliable tools for farmers. However, not all probiotics reduce foodborne diseases.
The bacteria most commonly used as animal-feed probiotics Include Lactobacillus, Bacillus, Streptococcus, Pediococcus, Enterococcus, and Bifidobacterium. Most commercially available probiotics contain more than one species for maximum effect. Some also contain fungi and yeast strains.
The live cultures used in probiotics are available in vegetative form and spore form. Vegetative cultures are humidity and heat-sensitive, while spore cultures are naturally strong when it comes to withstanding heat, antibiotics, and stomach acids.
Commercial Probiotic Products
Probiotic companies continue to roll out new probiotic products directed at the animal market. Here are some companies specializing in the animal-probiotic space.
One Company of France offers its spore-forming Bacillus subtilis Alterion probiotic strain targeting poultry. According to the company, “During production, birds are regularly challenged by various stress factors, including feed content or pathogens. By balancing gut microbiota, Alterion will help the animals to perform optimally, even under challenging conditions, providing at the same time a protection against future intestinal disorders.” Another company also recently announced the development of pipeline probiotics based on Bacillus.
The recently created U.S.-based company is developing a novel bacterial stain of Faecalibacterium prausnitzii for commercial livestock usage. The company is close to receiving Series funding to expand its research and development activities. The company recently partnered with Cornell University’s McGovern Center for Venture Development in the Life Sciences.
A company that develops pet probiotics recently launched a multistrain, high-count probiotic for companion dogs. The product is specifically developed to support canine digestive health. The product contains eight strains of bacteria and is cited as one of the highest bacteria count probiotics available for dogs. “We found many dog parents were giving their dogs probiotics designed for people without understanding their dog’s stomach is likely to be 10 to 100 times more acidic than the human stomach,” said Kamilah Lewis, senior director of marketing and communications of the company, in a press release. “We developed specifically for dogs as an alternative for consumers using human probiotics or canine probiotics that haven’t been effective. Each strain of bacteria was reviewed for its ability to tolerate stomach acid, low pH, and bile acids to make sure it travels through the dog’s GI tract and adheres to the intestinal wall.”
The Future of Animal Probiotics
High probiotic demand from cattle farmers and the companion animal sectors are expected to boost the adoption of advanced probiotic technologies, according to Grand View Research. Top players are investing hefty amounts in research and development to discover entirely novel products and applications. Nano encapsulated probiotics are expected to create numerous opportunities for the industry in near future.
Apart from being gut flora stabilizers, probiotics are expected to be utilized for more applications in animals, including for increased immunity and reduced stress levels. Probiotics will also continue to be used to improve the quality of eggs and meat and can reduce salmonella. And, overall, the microorganisms used in probiotics are approved for animal nutrition and do not constitute a major hazard for animal health. They do not affect the metabolic processes of animals as they are not transferred from the intestines to other body parts.
NSP Enzymes & Phytase Enzymes
The poultry feed contains Starch, proteins, fat and fibres. In these, the main components present in the feed made of cereals are cellulose, 1-3,1-4-ß-glucans and pentosanes of the arabinoxylan type. Because of the ability of the NSP enzymes which hydrolyze the components of cereals, a class of feed additives containing the NSP enzymes is mixed in the feed. These enzymes help the bird to hydrolyze the feed. About 0.00% to 9% increase in feed utilization in birds has been observed
From the day formers started using the feed enzymes mixture it has been observed that the growth of the bird has increased even more. In general soya bean meal and cornmeal are used for feeding Birds. As these are available at a very low cost, in Soya bean meal the level of non-starch polysaccharides is 29%. The exogenous enzymes with SBM and CM are effectively high in ducks when compared with chicken. It is also found that individual enzymes are showing good results than the cocktail of enzymes.
NSP Enzymes
Exogenous enzymes can hydrolyze the non-starch polysaccharides abundantly found in the feed given to birds. These carbohydrates cannot be digested by birds as they cannot produce enzymes, because of this reason the farmers have practised using the exogenous enzymes mixture, in other words, the cocktail of enzymes.
This cocktail contains enzymes like Xylanase, Amylase, and Protease. These enzymes are produced using microbial sources. The microbial sources used here are selected in such a way that they not only produce the enzyme but also, they act as Probiotics for birds. Care should be taken when the microbial source is selected are.
- The strain should be ecofriendly and should not be a parasite
- It should be suitable for the production of the Industrial scale
- It should grow with minimal nutrition
- Should produce higher amounts of enzyme
- The organisms used mainly for the production of enzymes are the fungal source
- Aspergillus spp (A. Niger)
- Penicillium spp
- Humicola spp. (H. insolens)
- Trichoderma spp
These are the largest group of enzyme-producing fungi. These organisms have a common thing among them that is they can produce enzymes that can break down various substances. Among them, the breakdown of the plant cell walls components mainly polysaccharides. The Bacterial source:
- Bacillus spp. (α-Amylase, proteases)
- B. licheniformis and B. subtilis
These are the largest group of bacteria used for the production of multiple enzymes and are also used as probiotics for birds.
Characteristics of Dietary Enzymes:
- The enzyme should withstand at the temperature of 40°C
- Should resist low pH
- Specific degradation site in the molecule
- Water content
- Presence of aerators / Inhibitors
- Substrate concentrations
Enzymes and their Actions:
- α-amylase: Endo-hydrolysis of α-1, 4-glucosidic linkages
- β- glucanase: Degrades β-glucan by cleaving β-1, 3(4) glucosidic linkages
- Pectinase: Degrades pectin α-1, 4-linked anhydrogalacuronic acid
- Xylanase: Hydrolysis of 1, 4-Beta-D-xylosidic linkages hemicellulose
Characteristics of Enzymes used in Animal Feed:
- Must act under acidic pH condition of the stomach
- Resist low pH
- Resist pepsin proteolytic action
- It should act in the digestive tract
Role of Poultry Enzymes:
- Poultry do not produce the enzyme for the hydrolyses of these non-starch polysaccharides (NSP) present in the cell wall of the grains
- The addition of exogenous enzymes specific for a given feed formulation will enhance the availability of feed components to the birds.
- Increase the energy by hydrolyzing the fibrous contents present in the feed.
- These exogenous enzymes act as endogenous enzyme supplements in conditions like more stress and at the early aged birds
- Calcium and phosphorus precipitations are prevented and absorption of them is promoted by these enzymes
- These exogenous enzymes will complement endogenous enzymes in few conditions at the early age of the birds.
- The production of endogenous enzymes may be limiting in conditions like age, health, climatic conditions etc. at times these exogenous enzymes help digestion in birds.
- Exogenous enzymes help the bird digest the NSP of the cell wall.
- As the NSP components of the cell wall break, the other useful components which are entrapped are also available to the birds
- The viscosity of the bird dropping decreases
- The intake of feed and its utilization increases, the intestinal flora is maintained properly
- There will be no Loss of endogenous proteins
- The starch in the cereals get unmasked as the cell wall breaks and the high amount of energy is produced to bird
- The proteins are also utilized up to the maximum extent and the maximum absorption of minerals and maximum utilization of feed nutrients is observed
Phytase Enzymes
Phytase is a digestive enzyme that releases plant phosphorus from Phytic acid.
Monogastric animals lack sufficient Phytase to release phosphorus. Adding extra Phytase to the diet increases phytate breakdown and consequent utilization of plant phosphorus.
If more phosphorus is available naturally, then less of this substance has to be added to the diet. This greatly reduces feed costs.
If phosphorus in the diet is utilized more efficiently, then less of this substance is excreted. This reduces the impact of livestock production on the environment.
These Phytase deliver exceptional phosphorus release in combination with outstanding reliability and consistency. They enable improved performance and greater business sustainability.
Phosphorus is an essential nutrient in livestock diets. It affects growth, reproduction and feed use.
Much of the naturally occurring phosphorus in feed ingredients is unavailable to animals. With most plant phosphorus being excreted undigested, inorganic phosphate has to be added to diets to compensate.
Increasing Phytic acid degradation and the availability of plant phosphorus can have major benefits for poultry and swine producers, including:
- Lower levels of inorganic phosphorus in diets
- Reduced feed costs
- Better sustainability of animal production
How does Animal affect the environment?
Livestock farming has a vast environmental footprint. It contributes to land and water degradation, biodiversity loss, acid rain, coral reef degeneration and deforestation. Nowhere is this impact more apparent than climate change – livestock farming contributes 18% of human-produced greenhouse gas emissions worldwide.
Why is phosphorus bad for the environment?
Too much phosphorus can cause increased growth of algae and large aquatic plants, which can result in decreased levels of dissolved oxygen– a process called eutrophication. High levels of phosphorus can also lead to algae blooms that produce algal toxins which can be harmful to human and animal health.
What are the environmental impacts of phosphorus in wastewater?
Too much nitrogen and phosphorus in the water causes algae to grow faster than ecosystems can handle. Significant increases in algae harm water quality, food resources and habitats, and decrease the oxygen that fish and other aquatic life need to survive.
Overview of Commercial Phytase, by Organism, Phytase Class, and Producing Company
| Donor Organism | Production Organism (host) | Phytase Class | Company |
| Aspergillus ficuum | Aspergillus niger | 3-phytase | BASF |
| Aspergillus niger | Trichoderma reesei | 3-phytase | AB Vista |
| Penicillium funiculosum | Penicillium funiculosum | 3-phytase | Adisseo |
| Peniophora lycii | Aspergillus oryzae | 6-phytase | Novozymes/ DSM |
| Escherichia coli | Schizosaccharomyces pombe | 6-phytase | DuPont |
| Escherichia coli | Pichia pastoris | 6-phytase | AB Vista |
| Escherichia coli | Trichoderma reesei | 6-phytase | AB Vista |
| Citrobacter braakii | Aspergillus oryzae | 6-phytase | Novozymes/ DSM |
| Buttiauxella sp. | Trichoderma reesei | 6-phytase | DuPont |
Optimum pH and temperature values of Phytase from various producers
| Microorganism | Optimum Temperature (°C) | Optimum pH | References |
| Aerobacter aerogenes | 27 | 6.8 | Greaves et al. (1967) |
| Arxula adeninivorans | 28 | 5.5 | Sano et al. (1999) |
| Aspergillus carneus | 30 | 6.0 | Ghareib (1990) |
| A. ficuum phytase A | 58 | 2.5 | Ullah and Sethumadhavan (1998) |
| A. ficuum phytase B | 63 | 2.5 and 5.0 | Ullah and Sethumadhavan (1998) |
| A. niger NRLL 3135 | 27 | 5.0 | Shieh and Ware (1968) |
| B. amyloliquefaciens | 70 | 7.5 | Boukhris et al. (2015) |
| Bacillus sp. DS 11 | 37 | 6.5 | Kim et al. (1998) |
| Enterobacter sp. 4 | 39 | 5.5 | Yoon et al. (1996) |
| E. coli | 37 | 7.5 | Greiner et al. (1993) |
| L. amylovorus | 37 | 6.0 | Sreeramulu et al. (1996) |
| L. plantarum | 30 | 6.0 | Saribuga et al. (2014) |
| L. edodes | 37 | 5.0 | Zhang et al. (2013) |
| Pichia rhodanensis | 28 | 6.0 | Nakamura et al. (2000) |
| R. oligosporus | 25 | 5.5 | Howson and Davis (1983) |
| Rothia mucilaginosa | 50 | 5.0 | Yu et al. (2015) |
| Schwanniomycess occidentalis | 30 | 6.5 | Segueilha et al. (1992) |
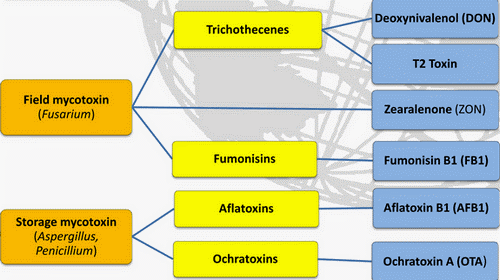
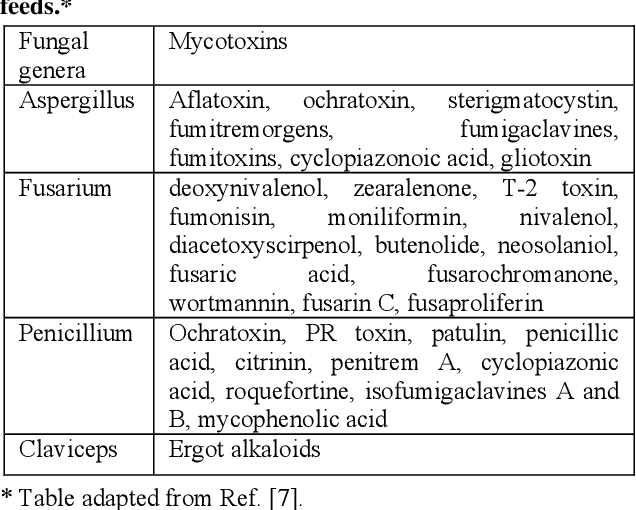
What are Mycotoxins?
Mycotoxins are toxic secondary metabolite compounds produced by mold or fungi. Mold can infect cannabis flowers as well as crops that go into the production of edibles and other cannabis products. Under the right conditions, mold can grow before plant harvest, during storage, or even directly on food items. Improper storage conditions create the perfect warm and humid environment for mold to flourish.
As mold grows and replicates, it naturally produces mycotoxins as metabolic byproducts. Unfortunately, many mycotoxins are relatively stable. This means they can remain chemically intact and toxic even after the harsh treatment of food processing.
Feed Ingradiants in Bad & Good Condition
Bad Condition


Good Condition

Examples
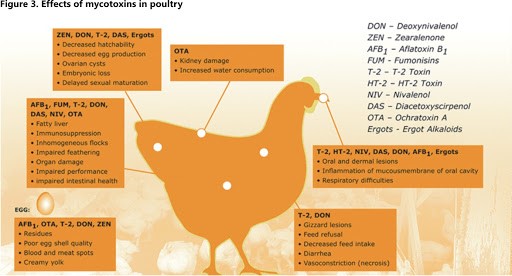
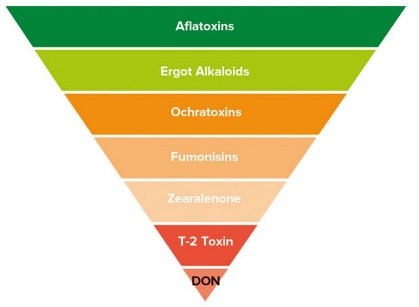
Aflatoxins are a family of compounds and represent one of the most noxious classes of mycotoxins. They’re produced by molds like Aspergillus that grow in soil and on grains.
Ochratoxin A, another mycotoxin, is produced by multiple species of Aspergillus and Penicillin. This is one of the most common food-contaminating mycotoxins.
Safety Risks
Mycotoxins are known to be severely toxic to humans, especially when exposure is chronic.
High levels of aflatoxin exposure, though very rare, can lead to an acute, fatal reaction. However, chronic exposure to low doses of aflatoxins causes damage to the liver and suppresses the immune system. Aflatoxins are carcinogenic and prolonged exposure can lead to an increased risk for liver cancer. Ochratoxin A can cause kidney damage and compromise the immune system.
These risks may be more pronounced in medical cannabis consumers who have pre-existing liver or kidney damage or are undergoing chemotherapy or radiation therapy.
Conclusion
Mycotoxins are a common plant and food contaminant that present unique health and safety challenges. But with high-quality cannabis testing, you can ensure that your products meet state regulations and are ready for your customers to safely consume.
Mycotoxin effect
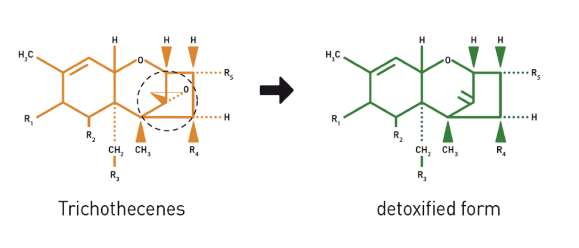

Aflatoxin B1
Aflatoxin B2
Organic Trace Minerals
What are organic trace minerals?
Organic trace minerals consist of these same metals being chelated, complexed or covalently bonded to amino acids, analogues of amino acids, proteins or organic acids in such a way that they tend to be more bioavailable in the intestine.

What are the 9 trace minerals?
Nine trace minerals (microminerals) are required by people in minute amounts:
- Chromium.
- Copper.
- Fluorine.
- Iodine.
- Iron.
- Manganese.
- Molybdenum.
- Selenium.
- Zinc
The Difference between Inorganic and Organic
Although the amount needed is measured in milligrams — one-thousandth of a gram or 0.00003527396 of an ounce — essential trace minerals are necessary to sustain life, fight disease and promote growth, development and healthy reproduction in livestock.
Trace minerals are an important part of raising healthy animals, and all living things need trace minerals to survive. To thrive in today’s modern animal production system, trace minerals must be added to an animal’s feed in such a way that the animal can take full advantage of the nutritional and health benefits they bring. Healthy animals contribute to a profitable and sustainable operation.
Comparing Inorganic and Organic Trace Minerals
Trace minerals come in three varieties: inorganic, organic and performance. These three mineral types vary at the most basic molecular level, and only one category provides consistent animal performance.
Inorganic Trace Minerals for Animals
The most basic and inexpensive trace minerals. Inorganic trace minerals are bonded to an inorganic molecule (one that doesn’t contain carbon) such as sulfate or oxide, making them relatively easy to produce and inexpensive to administer. Inorganic trace minerals are useful, but they can only do so much for your animal.
Organic Trace Minerals for Animals
Any trace mineral that is linked to a carbon-containing molecule is, by definition, organic, and organic trace minerals are more easily absorbed than inorganic. However, how well a trace mineral performs depends first on how strong and stable the link is to its carbon-containing molecule and secondly on how vital that molecule is to animal wellness.
Inorganic vs. Organic Trace Minerals
Inorganic and organic trace minerals are structurally different. Put simply, organic trace minerals are those whose metal is chemically bonded to a molecule-containing carbon. Inorganic minerals are relatively easy to produce, inexpensive to administer and are fed as a baseline portion of an animal’s diet. But an animal can’t optimally function on inorganics alone, because the number of inorganic minerals and animals can absorb is limited.
Trace Minerals Affect How Efficiently Animals use Vitamins
Trace minerals also play a role in the retention and stability of fat-soluble vitamins A (known as retinol), E and D3. All three play a critical role in animal immune systems.
If fat-soluble vitamins are reduced in diets due to shortages or price spikes, using inorganic trace minerals will make the problem worse by degrading vitamin activity during storage, according to a University of Minnesota study (Shurson et al., 2011).
Contact Us
Have questions or need help? Use the form to reach out and we will be in touch with you as quickly as possible.