Human Healthcare Hygiene and Biocide Products
- UVC light Disinfection
- Chemicals Disinfectant and Sterilant
UVC light Disinfection
Overview
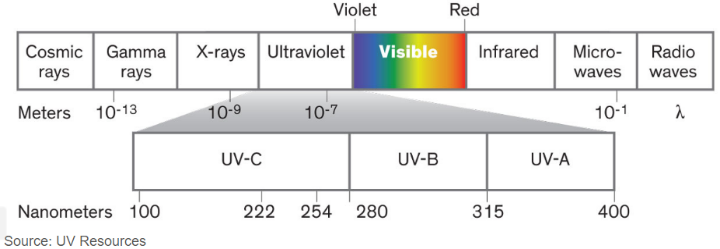
Ultraviolet (UV) radiation covers the wavelength range of 100–400 nm, which is a higher frequency and lower wavelength than visible light. UV radiation comes naturally from the sun, but it can also be created by artificial sources used in industry, commerce and recreation. The UV region covers the wavelength range of 100-400 nm and is divided into three bands:
- UVA (315-400 nm)
- UVB (280-315 nm)
- UVC (100-280 nm)
As sunlight passes through the atmosphere, all UVC and approximately 90% of UVB radiation are absorbed by ozone, water vapour, oxygen and carbon dioxide. UVA radiation is less affected by the atmosphere. Therefore, the UV radiation reaching the Earth’s surface is largely composed of UVA with a small UVB component. The amount of UV radiation from the sun that hits the Earth’s surface depends on several factors, including the sun’s height in the sky, latitude, cloud cover, altitude, the thickness of the ozone layer and ground reflection. Reductions in the ozone layer due to human-created pollution increase the amount of UVA and UVB that reaches the surface. This can impact human health, animals, marine organisms and plant life. In humans, increased UV exposure can cause skin cancers, cataracts and immune system damage. Ultraviolet light in the C-band (UV-C) is known for its lethal effect against micro-organisms such as viruses, bacteria, fungi, yeast and other harmful microbes.



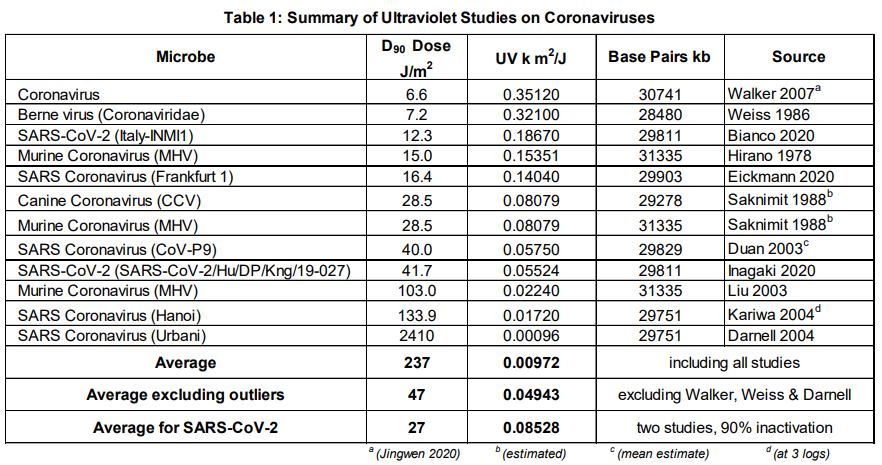

Ultraviolet light in the C-band (UV-C) is known as Ultraviolet Germicidal Irradiation (UVGI), due to its lethal effect against micro-organisms such as viruses, bacteria, fungi, yeast and other harmful microbes that pose a threat to human health. The table below shows the UV-C dosage required to treat a wide range of coronaviruses and demonstrates that UVGI is effective for surface and air treatment to prevent and control the spread of this highly infectious disease.
UV Light Might Keep the World Safe from the Coronavirus—and Whatever Comes Next
Notable UV Sterilizers use for various indoor and outdoor objects
Ultraviolet sterilization technology is used to kill the bacteria on the articles. After authority detection, irradiation for a certain time can effectively kill all kinds of viruses, E. Coil, Candida Albicans, staphylococcus aureus and other common bacteria, with bactericidal efficiency of up to 99.99%. PuraHub Limited marketed many kinds of UVC disinfection products to help people all over Bangladesh getting healthy light. The high standard of product production and high quality of the product is one of the most attractable factors for customers. Our products certified by ISO9001:2015 and products certified by UL, DLC, FCC, TUV, CE, ROHS, SAA, PSE etc. What we insist…
“Quality First, Customer First” and keep innovation for establishing long-term cooperation with our clients. Trust us, make your wise decision today!
Chemicals Disinfectant and Sterilant



According to estimates from the Centers for Disease Control and Prevention, healthcare-associated infections (HAIs) kill more people each year globally than car accidents, breast cancer or Aids, and cost the US healthcare system an estimated $30bn-$45bn each year. HAIs can develop more than 48-72 hours after patient admission and up to ten days after hospital discharge, and hospitals, community clinics, nursing homes and centres handling outpatient surgery, dialysis and rehabilitation are all possible breeding grounds for multiple drug-resistant microbes. The most common HAIs are:
- Central line-associated bloodstream infections
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Vancomycin-resistant Enterococci bloodstream infections
- Clostridium difficile
- Surgical site infections (SSIs)
Chemical sterilants and disinfectants are the most important defence against HAIs. Prevention strategies may range from hand hygiene using antiseptics and low-level disinfectants to the sterilisation of gastro-intestinal endoscopes using potent, high-level disinfectants. Even environmental cleanliness with phenols and bleach solutions may help prevent fatal HAIs such as Clostridium difficile. To help healthcare facilities decide on the disinfectants and disinfection procedures that best fit their needs, Global Data has profiled the top ten disinfection solutions.
Sterilants and high-level disinfectants
Formaldehyde

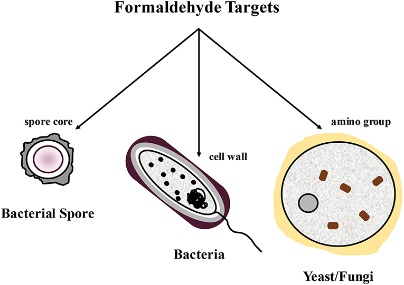
Formaldehyde – Primarily available as a water-based solution called formalin, which contains 37% formaldehyde by weight – is used as a high-level disinfectant and sterilant. Formaldehyde exerts its bactericidal, tuberculocidal, fungicidal, virucidal and sporicidal effects in the aqueous state, as well as in combination with low-temperature steam. This extremely reactive chemical’s mechanism of action is attributed to its interactive and cross-linking properties with protein, DNA and RNA in vitro, resulting in the disruption of DNA synthesis. It can also penetrate bacterial spores.
Formaldehyde has been traditionally used to sterilize equipment such as surgical instruments and haemodialysers in combination with alcohols. Paraformaldehyde, a solid polymer of formaldehyde, is used in combination with low-temperature steam for the disinfection of heat-sensitive medical equipment. Exposure hazards and the potential carcinogenic effects of formaldehyde have limited its healthcare uses recently, while next-generation aldehydes such as glutaraldehyde and o-phthalaldehyde, with better sporicidal activity, faster onset of action and lesser exposure effects, have replaced formaldehyde in the disinfection manuals of most healthcare establishments.
Glutaraldehyde
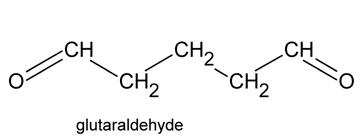

Glutaraldehyde is a saturated dialdehyde widely used as a potent sterilant and high-level disinfectant. Its broad spectrum, covering bactericidal, sporicidal, fungicidal and virucidal activity, makes it an ideal chemical for the low-temperature disinfection and sterilisation of critical and semi-critical equipment such as endoscopes, dialysers and surgical tools. Aqueous solutions of glutaraldehyde when activated (rendered alkaline at 7.5- 8.5 PH) become sporicidal, while its microbicidal action is attributed primarily to its strong association with the outer layers of bacterial cells and the alkylation of sulphydryl, hydroxyl, carboxyl and amino groups, which alter RNA, DNA and protein synthesis within microorganisms.
Glutaraldehyde is a saturated dialdehyde that has gained wide acceptance as a high-level disinfectant and chemical sterilant. Aqueous solutions of glutaraldehyde are acidic and generally in this state are not sporicidal. Only when the solution is “activated” (made alkaline) by the use of alkalinising agents to PH 7.5–8.5 does the solution become sporicidal. Once activated, these solutions have a shelf-life of minimally 14 days because of the polymerization of the glutaraldehyde molecules at alkaline PH levels. This polymerization blocks the active sites (aldehyde groups) of the glutaraldehyde molecules that are responsible for its biocidal activity.
Glutaraldehyde is also mycobactericidal, and glutaraldehyde formulations with phenols and alcohols have better usage life while maintaining excellent microbicidal activity. Glutaraldehyde exposure should be monitored to ensure a safe working environment. Acute or chronic exposure, resulting in skin irritation, mucous membrane irritation and multiple pulmonary symptoms such as occupational asthma and allergic rhinitis have been reported in healthcare workers. Due to its exposure hazards, US healthcare associations advocate the use of glutaraldehyde alternatives such as o-phthalaldehyde, hydrogen peroxide and peracetic acid.
Ortho-phthalaldehyde
Since its introduction in 1999, ortho-phthalaldehyde (OPA) has been accepted as a better, safer alternative to glutaraldehyde in most US healthcare facilities. OPA by Advanced Sterilization Products was cleared by the US FDA as a high-level disinfectant and emerged as a suitable replacement of glutaraldehyde for the disinfection of endoscopes. OPA has excellent microbiocidal activity and superior mycobactericidal activity compared with glutaraldehyde and has potent bactericidal and sporicidal activity. Like glutaraldehyde, it interacts with amino acids, proteins and microorganisms.
OPA has many advantages over glutaraldehyde, such as improved stability at varying PH ranges, lower inhalation exposure risk and a wider range of material compatibility, although it costs almost three times more; but, considering the cost of the sophisticated ventilation systems needed to minimize the respiratory hazards of using glutaraldehyde, OPA is more economical. Ortho-phthalaldehyde is a high-level disinfectant that received FDA clearance in October 1999. It contains 0.55% 1,2-benzenedicarboxaldehyde (OPA). OPA solution is a clear, pale-blue liquid with a PH of 7.5.
Hydrogen peroxide


Disinfectant solutions containing 7.5% hydrogen peroxide have been approved by the US FDA for sterilisation and high-level disinfection in healthcare settings. “Disinfectant solutions containing 7.5% hydrogen peroxide have been approved by the US FDA for sterilisation.” A 0.5% accelerated hydrogen peroxide demonstrated bactericidal and virucidal activity in 1 minute and mycobactericidal and fungicidal activity in 5 minutes.
Its good, broad-spectrum bactericidal, virucidal, sporicidal and fungicidal properties, combined with its excellent stability and environmentally friendly characteristics, have made hydrogen peroxide the disinfectant of choice for semi-critical and non-critical equipment while being an ideal surface disinfectant. Despite not being listed as equipment-compatible by all endoscope manufacturers, hydrogen peroxide-based disinfectant solutions are still considered ideal alternatives for other toxic sterilants, such as ethylene oxide and aldehyde disinfectants. Hydrogen peroxide produces destructive hydroxyl-free radicals that act on membrane lipids, DNA and other essential cell components.
Peracetic acid
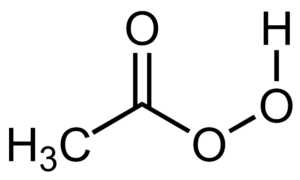
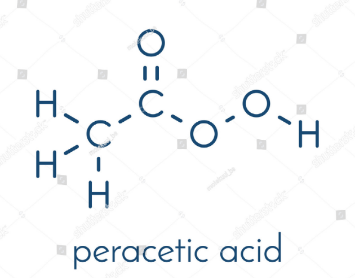
Peracetic, or peroxyacetic, acid is characterized by rapid action against all microorganisms. Special advantages of peracetic acid are that it lacks harmful decomposition products (i.e., acetic acid, water, oxygen, hydrogen peroxide), enhances the removal of organic material, and leaves no residue. It remains effective in the presence of organic matter and is sporicidal even at low temperatures. Peracetic acid can corrode copper, brass, bronze, plain steel, and galvanized iron but these effects can be reduced by additives and PH modifications. It is considered unstable, particularly when diluted; for example, a 1% solution loses half its strength through hydrolysis in 6 days, whereas 40% peracetic acid loses 1%–2% of its active ingredients per month.
Another emerging alternative to ethylene oxide and aldehyde sterilants is peracetic acid. Peracetic acid-based solutions are considered to be a more potent disinfectant than hydrogen peroxide; are sporicidal, bactericidal, virucidal and fungicidal at low concentrations, and are environment friendly. They have replaced traditional disinfectants for medical devices, endoscopes and haemodialysers. It also acts as an environmental surface sterilant, and behaves similarly to other oxidising agents, disrupting cell wall permeability and oxidising sulphhydryl and sulphur bonds in proteins, enzymes and other metabolites.
Hydrogen peroxide and Peracetic acid combination


Two chemical sterilants are available that contain peracetic acid plus hydrogen peroxide (i.e., 0.08% peracetic acid plus 1.0% hydrogen peroxide and 0.23% peracetic acid plus 7.35% hydrogen peroxide. Peracetic acid, when combined with hydrogen peroxide, was found to be more effective, typically against glutaraldehyde-resistant mycobacteria. Combination of products cleared by the FDA. The combination of peracetic acid and hydrogen peroxide has been used for disinfecting haemodialysers, but the mix was found to be incompatible with flexible gastrointestinal endoscopes as claimed by leading US manufacturers.
Intermediate-level disinfectants
Sodium hypochlorite
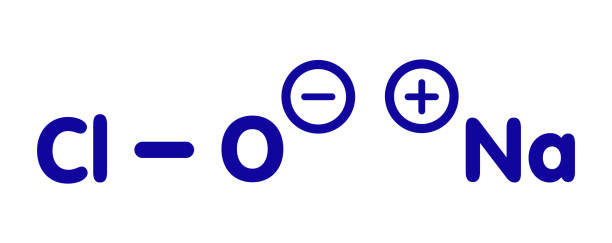
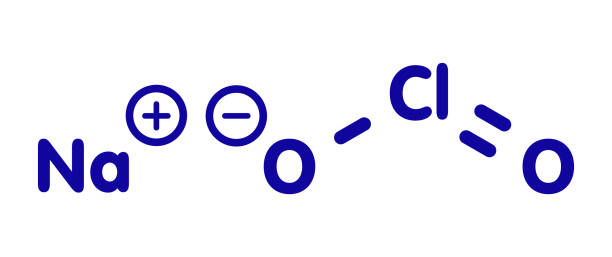



Chlorine-releasing agents (CRAs), the most popular sodium hypochlorite solution, are widely used for the disinfection of hard surfaces and blood spillages containing the human immunodeficiency virus or hepatitis B virus. Recently, sodium hypochlorite was designated as the best defence against hospital-acquired and community-acquired Clostridium difficile infections. With a broad spectrum of antimicrobial activity, sodium hypochlorite is inexpensive with low toxicity. CRAs are highly active oxidising agents and destroy the cellular activity of proteins. Hypochlorous acid, the active moiety, has been shown to have harmful effects on bacterial DNA through the formation of chlorinated derivatives of nucleotide bases.
High concentrations of sodium hypochlorite display significant levels of sporicidal and virucidal activity. Sodium hydroxide (7681-52-9)- 15-30%. Sodium hypochlorite (1310-73-2)-3-5%. The most prevalent chlorine products in the United States are aqueous solutions of 5.25%–6.15% sodium hypochlorite (see glossary), usually called household bleach. They have a broad spectrum of antimicrobial activity, do not leave toxic residues, are unaffected by water hardness, are inexpensive and fast-acting, remove dried or fixed organisms and biofilms from surfaces and have a low incidence of serious toxicity. After reviewing environmental fate and ecologic data, EPA has determined the currently registered uses of hypochlorites will not result in unreasonable adverse effects on the environment.
Iodophors
Iodophors, complexes of iodine and a solubilising agent or carrier, are used as antiseptics and surface disinfectants. Iodine has bactericidal, fungicidal, tuberculocidal, virucidal and sporicidal properties, while its antiseptic properties are well known.
Iodophors, such as povidone-iodine and poloxamer-iodine, are much more stable, have fewer irritant characteristics and exert better microbicidal action than aqueous iodine solutions. The FDA has not cleared any liquid chemical sterilant or high-level disinfectant with iodophors as the main active ingredient, but iodophors are still used in healthcare settings for disinfecting blood culture bottles and medical equipment such as thermometers.
Low-level disinfectants
Phenols

“Phenolic disinfectants disrupt the cell membrane of microorganisms.” Since Dr Joseph Lister’s use of phenols for his pioneering work on antiseptic surgery, phenolic disinfectants have been used as low and intermediate-level disinfectants. Phenolic disinfectants are effective bactericides, fungicides, tuberculocides and virucides, but are ineffective against spore-forming bacteria such as Clostridium difficile.
EPA-registered phenolic disinfectants are used to disinfect surface areas and non-critical medical devices. Phenolics are not FDA-cleared as high-level disinfectants for use with semi-critical items. Phenolic disinfectants disrupt the cell membrane of microorganisms, and two phenol derivatives used commonly in hospital disinfectants are orthophenylphenol and ortho-benzyl-parachlorophenol.
Quaternary ammonium compounds
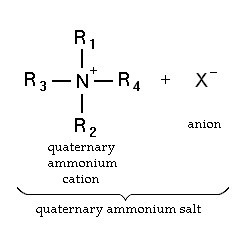
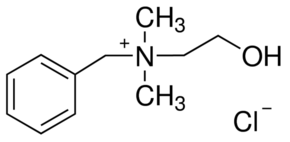
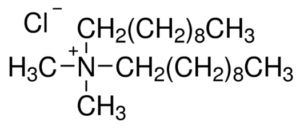
Quaternary ammonium compounds (QACs) are used for a variety of clinical purposes such as preoperative disinfection, disinfection of non-critical instruments, and hard-surface cleaning and deodorisation. QACs possess bactericidal, fungicidal and virucidal properties; however, they only display mycobacteria static and spore static activity. Quaternary ammonium disinfectants contain NH4+, the strong positive charge that results in better contact with negatively charged surfaces, making them good cleaning agents. EPA-registered QACs are appropriate for use in disinfecting medical equipment that contacts unbroken skin, such as blood pressure cuffs. US hospitals also use quaternary ammonium-based disinfectants for the terminal disinfection of hospital rooms.
Chemically, the quaternary is organically substituted ammonium compounds in which the nitrogen atom has a valence of 5, four of the substituent radicals (R1-R4) are alkyl or heterocyclic radicals of a given size or chain length, and the fifth (X‑) is a halide, sulfate, or similar radical. Each compound exhibits its own antimicrobial characteristics, hence the search for one compound with outstanding antimicrobial properties. Some of the chemical names of quaternary ammonium compounds used in healthcare are alkyl dimethyl benzyl ammonium chloride, alkyl didecyl dimethyl ammonium chloride, and dialkyl dimethyl ammonium chloride. The newer quaternary ammonium compounds (i.e., fourth-generation), referred to as twin-chain or dialkyl quaternary (e.g.) didecyl dimethyl ammonium bromide and dioctyl dimethyl ammonium bromide purportedly remain active in hard water and are tolerant of anionic residues. A few case reports have documented occupational asthma as a result of exposure to benzalkonium chloride.
Table:1 Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants
| Chemical Characteristics
| Hydrogen Peroxide (7.5%) | Peracetic Acid (0.2%) | Glutaraldehyde (≥2.0%) | OPA (0.55%) | Hydrogen Peroxide / Peracetic Acid (7.35%/0.23%) |
| High-level disinfectant claim | 30 minutes @ 20°C | Not Applicable | 20-90 minutes @ 20o-25°C | 12 minutes @ 20°C, 5 minutes @ 25°C in AER | 15 minutes @ 20°C |
| Sterilization Claim | 6 hours @ 20°C | 12 minutes @ 50-56°C | 10 hours @ 20o-25°C | None | 3 h @ 20°C |
| Activation | No | No | Yes (alkaline glutaraldehyde) | No | No |
| Reuse life (number of days a product can be reused as determined by re-use protocol) | 21 days | Single-use | 14-30 days | 14 days | 14 days |
| Shelf-life stability (the time a product can remain in storage (unused)) | 2 years | 6 months | 2 years | 2 years | 2 years |
| Disposal Restrictions | None | None | Local (no U.S. EPA regulations exist but some states and local authorities have disposal restrictions) | Local (no U.S. EPA regulations exist but some states and local authorities have disposal restrictions) | None |
| Materials Compatibility | Good | Good | Excellent | Excellent | No data |
| Monitor MEC of solution | Yes (6%) | No | Yes (1.5% or higher) | Yes (0.3% OPA) | No |
| Safety | Serious eye irritant (safety glasses) | Serious eye and skin irritant (conc. Soln.) 5 | Respiratory irritant | Eye irritant, stains skin | Eye irritant |
| Processing | Manual or automated | Automated | Manual or automated | Manual or automated | Manual |
| Organic material resistance | Yes | Yes | Yes | Yes | Yes |
| OSHA exposure limit | 1 ppm TWA | None | None (The ceiling limit recommended by the American Conference of Governmental Industrial Hygienists is 0.05 ppm.) | None | Hydrogen Peroxide -1 ppm (time-weighted average for a conventional 8-hour workday) |
| Cost profile (per cycle)1 | + (manual) ++ (automated) | +++++ (automated) | + (manual) ++ (automated) | ++ (manual) | ++ (manual) |
Abbreviations and Footnotes: OPA ortho-phthalaldehyde (FDA cleared as a high-level disinfectant, included for comparison to other chemical agents used for high-level disinfection). AER Automated Endoscope Reprocessor, MEC minimum effective concentration is the lowest concentration of active ingredients at which the product is still effective.
1 per cycle cost profile considers the cost of the processing solution (suggested list price to healthcare facilities in August 2001) and assumes maximum use life (e.g., 21 days for hydrogen peroxide, 14 days for glutaraldehyde), 5 reprocessing cycles per day, 1-gallon basin for manual processing, and 4-gallon tank for automated processing.
+ = least expensive; +++++ = most expensive
Table 2: Summary of advantages and disadvantages of chemical agents used as chemical sterilants1 or as high-level disinfectants
| Sterilization Method
| Advantages | Disadvantages |
| Peracetic Acid/Hydrogen Peroxide |
|
|
| Glutaraldehyde |
|
|
| Hydrogen Peroxide |
|
|
| Ortho-phthalaldehyde |
|
|
| Peracetic Acid |
|
|
Modified from Rutala
1. All products effective in presence of organic soil, relatively easy to use, and have a broad spectrum of antimicrobial activity (bacteria, fungi, viruses, bacterial spores, and mycobacteria). The above characteristics are documented in the literature; contact the manufacturer of the instrument and sterilant for additional information. All products listed above are FDA-cleared as chemical sterilants except OPA, which is an FDA-cleared high-level disinfectant.

Top 5 Chemical Disinfectants Used in Hospitals
Choosing the right cleaning product for patient room sanitation can be a complicated process. You need to consider the advantages and drawbacks of each cleaning chemical. Stringent disinfection reduces the risk of healthcare-associated infections (HAIs). Currently, there are five main EPA-registered chemicals that hospitals use for disinfectants: Quaternary Ammonium, Hypochlorite, Accelerated Hydrogen Peroxide, Phenolics, and Peracetic Acid.
Quaternary Ammonium
Quaternary ammonium compounds are used broadly in routine cleaning. The Centers for Disease Control and Prevention considers quaternary to be a low-level disinfectant effective against most bacteria, enveloped viruses, and some fungi. It’s used in products and is compatible with most hard surfaces. Quaternary ammonium products are best used on non-critical surfaces such as floors, bed-rails, tray tables, blood pressure cuffs, walls, and partitions.
Hypochlorite
Hypochlorites are the most commonly used chlorine disinfectants. Sodium Hypochlorite is commercially available as household bleach. This EPA-registered chemical is stable and fast-acting. While generally considered safe, bleach can cause skin and eye irritation. It is corrosive to the metal in high concentrations and can discolour fabric. Hypochlorites effectively kill bacteria, fungi, and viruses. Hospitals can use these products for bathrooms, food prep zones, and blood spills. All areas must be pre-cleaned to remove organic matter before disinfection. When using concentrated products, follow strict dilution protocols.
Phenolics
Phenolics have been around for a long time. Sir Joseph Lister used a phenol called carbolic acid as a surgery antiseptic in the 1800s. The antimicrobial properties of phenol derivatives have improved over time. Phenolics are present in hospitals today. These products are best for the disinfection of non-porous surfaces and non-critical devices. Use phenolics with care and follow manufacturers recommendations carefully because improper preparations can be dangerous to newborns. Remember, product residue can irritate the skin.
Peracetic Acid
Peracetic acid preparations are rapid-acting disinfectants. They are bactericidal, fungicidal, virucidal, mycobactericidal, and sporicidal. However, Peracetic acid can become unstable when diluted. It can corrode some metals such as copper and brass. Hospitals used Peracetic acid in automated machines to sterilize medical instruments and to disinfect hemodialyzers.
Accelerated Hydrogen Peroxide
Accelerated Hydrogen Peroxide (AHP) is a more recent breakthrough in hospital disinfectants. These products are a blend of safe, active cleaning agents with hydrogen peroxide. These compounds are safe for the cleaning staff and the environment with the lowest EPA toxicity category of IV. These one-step cleaners disinfect in the presence of organic matter and blood. They are efficient with short dwell times. AHP kills bacteria, viruses, mycobacteria, pathogenic fungi, and blood-borne pathogens.
Choosing the Right Disinfectant
Hospitals should carefully consider the right cleaning product for the job. A meta-analysis published by the Agency for Healthcare Research and Quality identifies the most effective environmental cleaning methods for the prevention of HAIs. The analysis notes that an effective disinfection protocol should consider these five factors:
- Targeted microbes (hepatitis, HIV, C. Difficile, etc.)
- Surface type (cloth, metal, plastic, etc.)
- Disinfectant compatibility with surfaces and materials
- Cost and ease of use
- Safety of staff and patients
Selecting cleaning chemicals involves multiple stakeholders at the facility, including infection control committees and environmental services. Hospital staff should work together to choose the right disinfectant for each job.
No-Touch Modalities
Two kinds of devices have been developed and commercially produced to disinfect hospital rooms. One type of device emits UV light, and another produces a mist or vapour of hydrogen peroxide. These devices are often referred to as no-touch or automated modalities because they disinfect via a stand-alone machine instead of the manual application of chemical agents. Experts indicate that no-touch modalities should be used only as adjunctive infection control measures.
Ultraviolet Light
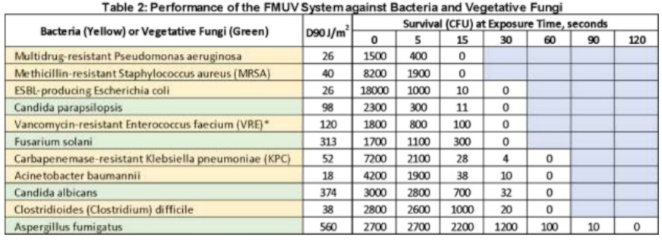
The use of a UV wavelength light as a no-touch, automated modality for hospital room disinfection has received significant recent attention. The UV-C wavelength of 200 to 270 nanometers is germicidal and involves breaking of molecular bonds in DNA, resulting in microorganism death. Advantages of UV-C technology include its microbicidal activity against a wide range of healthcare-associated pathogens, including C. difficile, and the ability for more rapid room decontamination compared to hydrogen peroxide systems. Automated UV-C systems have most commonly been tested for post-discharge terminal disinfection in hospital rooms of patients with C. difficile infection.
This technology’s disadvantages include the requirement for the room to be vacated and disinfected before decontamination, its use only for terminal disinfection (vs. daily disinfection), and its significant cost. Also, equipment and furniture must be moved away from walls to prevent shadowing because UV-C systems cannot disinfect areas without a direct or indirect line of sight.
Finally, these units require significant time for effective disinfection and can therefore adversely affect bed turnover time. While dependent on many factors (e.g., system being used, dose, the organism being targeted), the turnaround time for these devices can range from approximately 15 to 20 minutes for vegetative bacteria to approximately 50 to 100 minutes for C. difficile spores. A recent study utilizing a UV-reflective wall coating resulted in significantly decreased decontamination times, from ∼25 minutes to ∼3 minutes for MRSA, and from ∼43 minutes to ∼9 minutes for C. difficile spores.
Hydrogen Peroxide-Producing Systems
The use of hydrogen peroxide-producing systems for disinfecting hospital room surfaces and objects have been recently studied. Several systems that produce hydrogen peroxide using different methods are available (e.g., dry mist, hydrogen peroxide vapour). Advantages of these include reliable microbicidal activity against a variety of pathogens associated with HAIs, including C. difficile, as well as uniform distribution in the room via an automated dispersal system, such that furniture and equipment do not need to be moved away from walls.
However, as with UV-C devices, all patients and health care staff must leave the room before decontamination, and these devices are used for terminal room disinfection (i.e., not for daily disinfection). Costs of these devices can also be substantial, and a lot of time is required for effective disinfection. High-level training is required to operate these devices. Air vents, doors, and windows must be isolated and sealed, and active monitoring with sensors is necessary to monitor for leaks and ensure that the room is safe for personnel to enter. A safety concern with improper use is airway and mucous membrane irritation. As with UV-C devices, hydrogen peroxide–producing systems are a relatively recent disinfection technology and, pending further studies, are not yet routinely used for disinfecting hospital rooms.