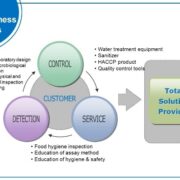
Food safety
Access to sufficient amounts of safe and nutritious food is key to sustaining life and promoting good health. Unsafe food containing harmful bacteria, viruses, parasites, or chemical substances can cause more than 200 different diseases – ranging from diarrhoea to cancers. Around the world, an estimated 600 million – almost 1 in 10 people – fall ill after eating contaminated food each year, resulting in 420 000 deaths and the loss of 33 million healthy life years.
Food safety, nutrition, and food security are closely linked. Unsafe food creates a vicious cycle of disease and malnutrition, particularly affecting infants, young children, the elderly, and the sick. In addition to contributing to food and nutrition security, a safe food supply also supports national economies, trade, and tourism, stimulating sustainable development. The globalization of food trade, a growing world population, climate change and rapidly changing food systems have an impact on the safety of food. WHO aims to enhance at a global and country-level the capacity to prevent, detect, and respond to public health threats associated with unsafe food.
What are the common chemicals used in cleaning and sanitizing?
- CHLORINE. Chlorine is the most common chemical sanitizing agent used in the milk industry.
- IODINE. Iodine sanitisers used in milk plants are usually in the form of Iodophors.
- QUATERNARY AMMONIUM COMPOUNDS (Cationic).
- CHLORINE DIOXIDE.
- ACID SANITIZERS.
What chemicals disinfect service area?
5 Most Effective Sanitizing Chemicals
- Hypochlorites – Probably the most commonly found chemical in sanitisers, hypochlorite and its compounds are highly effective in killing microbes. Not only that, but they can also successfully destroy microbe DNA. However, spores do have a resistance to hypochlorites because their outer coat is not susceptible to the oxidizing nature of the chemical.
- Chlorine Dioxide – Although known as explosive in its gas form, this inorganic compound is safe in a liquid solution. It is highly effective against bacteria, viruses and fungi as it reacts with proteins and fatty acids to break down the organisms structurally. It is effective in low concentrations, with just a 5ppm solution enough to adequately sanitize food contact surfaces with a contact time of less than 1 minute.
- Iodophors – Effective as both a sanitiser and a disinfectant, the real advantage that Iodophors have over other chemicals is their sustained-release effect. It means that microbes are killed steadily over an extended period of time, ensuring a surface stays clean and sanitized for longer. Iodophors work by attaching themselves to sulfurs in proteins, which basically warrants them inactive.
- Peroxyacetic Acid (PAA) – Effective against most microorganisms, it can work very well in colder temperatures. Especially when they are paired with stabilized hydrogen peroxide. Even at temperatures as low as 40C, it records high levels of microbe mortality, which in turn makes it ideal in food processing areas where lower-than-ambient temperatures are necessary. PAA is also effective against biofilms but is less effective as water PH nears neutral and depending on requirements, can be used at a concentration from 100-200 ppm (peroxyacetic acid) and 80-600 ppm (hydrogen peroxide).
- Quaternary Ammonium Compounds (QACs or Quats) – One of the more complex compounds found in sanitisers, QACs comprise nitrogen bound to 4 different organic groups. They work by blocking a microbe’s nutritional intake process, effectively starving them, but it has no real effect on spores. As sanitisers, they are usually applied at concentrations of 200 ppm and allowed to dry, at which point the QAC residue continues to work. They are usually odourless, non-staining, non-corrosive and relatively non-toxic to users and while heavy soil and hard water can severely lessen effectiveness, chelating agents can be added to some compounds to counter these issues.
10 Tips: Be Food Safe
A critical part of healthy eating is keeping foods safe. Individuals in their own homes can reduce contaminants and keep food safe to eat by following safe food handling practices. Four basic food safety principles work together to reduce the risk of foodborne illness — Clean, Separate, Cook, and Chill. These four principles are the cornerstones of Fight BAC!®, a national public education campaign to promote food safety to consumers and educate them on how to handle and prepare food safely.
CLEAN
- Wash hands with soap and water: Wet hands with clean running water and apply soap. Use warm water if it is available. Rub hands together to make a lather and scrub all parts of the hand for 20 seconds. Rinse hands thoroughly and dry using a clean paper towel. If possible, use a paper towel to turn Off the faucet.
- Sanitize surfaces: Surfaces should be washed with hot, soapy water. A solution of 1 tablespoon of unscented, liquid chlorine bleach per gallon of water can be used to sanitize surfaces.
- Clean sweep refrigerated foods once a week: At least once a week, throw out refrigerated foods that should no longer be eaten. Cooked leftovers should be discarded after 4 days; raw poultry and ground meats, 1 to 2 days.
- Keep appliances clean: Clean the inside and the outside of appliances. Pay particular attention to buttons and Handles where cross-contamination to hands can occur.
- Rinse produce: Rinse fresh vegetables and fruits under running water just before eating, cutting, or cooking. Even if you plan to peel or cut the produce before eating, it is important to thoroughly rinse it first to prevent microbes from transferring from the outside to the inside of the produce.
SEPARATE
- Separate foods when shopping: Place raw seafood, meat, and poultry in plastic bags. Store them below ready-to-eat foods In your refrigerator.
- Separate foods when preparing and serving: Always use a clean cutting board for fresh produce and a separate one for raw seafood, meat, and poultry. Never place cooked food back on the same plate or cutting board that previously held raw food.
COOK AND CHILL
- Use a food thermometer when cooking: A food thermometer should be used to ensure that food is safely cooked and that cooked food is held at safe temperatures until eaten.
- Cook food to safe internal temperatures: One effective way to prevent illness is to check the internal temperature of seafood, meat, poultry, and egg dishes. Cook all raw beef, pork, lamb, and veal steaks, chops, and roasts to a safe minimum internal temperature of 145 °F. For safety and quality, allow the meat to rest for at least 3 minutes before carving or eating. Cook all raw ground beef, pork, lamb, and veal to an internal temperature of 160 °F. Cook all poultry, including ground turkey and chicken, to an internal temperature of 165 °F.
- Keep foods at safe temperatures: Hold cold foods at 40 °F or below. Keep hot foods at 140 °F or above. Foods are no longer safe to eat when they have been in the danger zone between 40-140 °F for more than 2 hours (1 hour if the temperature was above 90 °F).
PuraHub Limited marketed HALAL certified approved company’s products


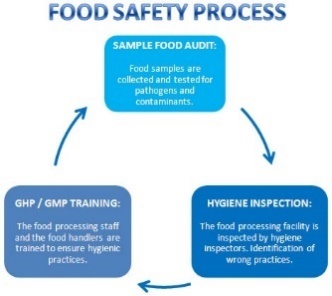

FOOD SAFETY PROCESS and HACCP PLAN (Hygiene System and Equipment)
Food, Beverage and Dairy Hygienic plants
Sanitation in Meat and Poultry Plants
Cleaning steps of the CIP (Cleaning in Place) process in dairy plants
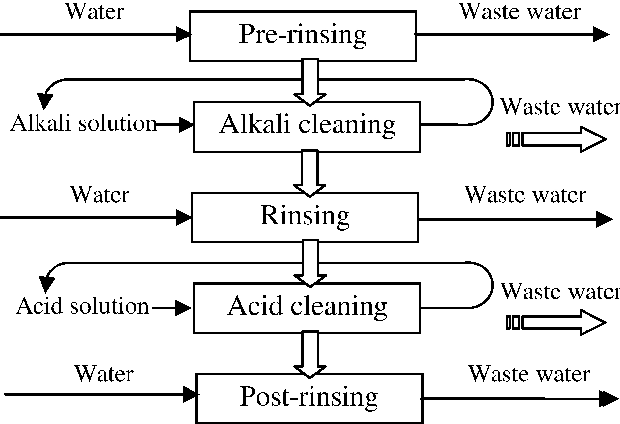
Automated & efficient cleaning of dairy, juice or drink production equipment
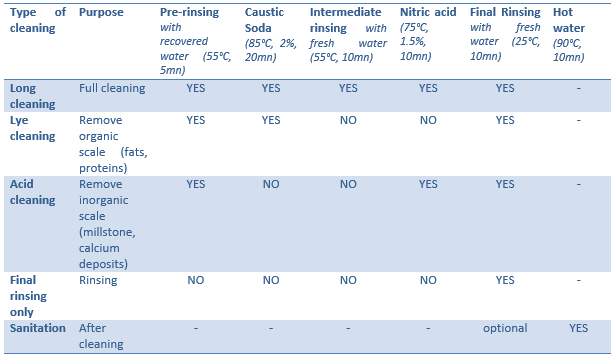
Industrial hygiene and cleaning


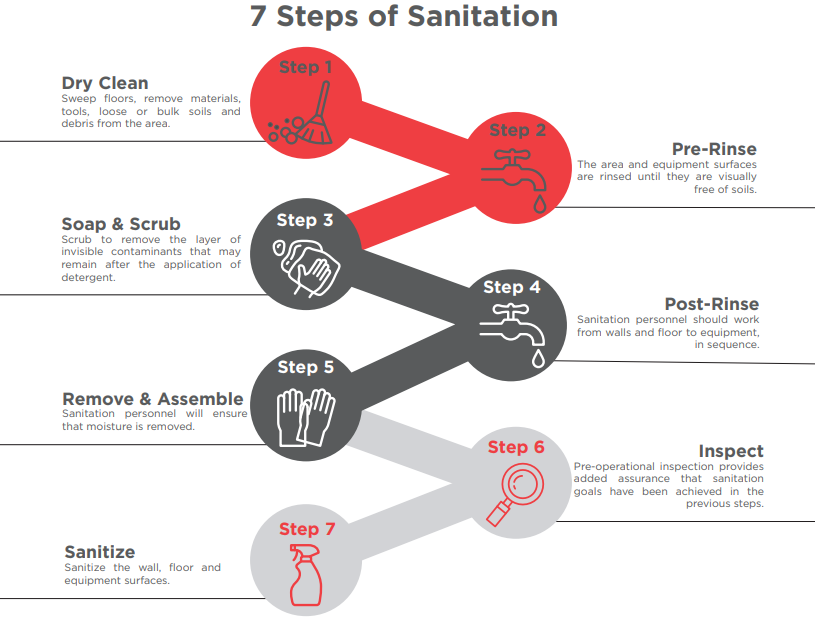
7 Steps of Effective Sanitation
PuraHub Limited cleaning products for the food industry
Formaldehyde


Formaldehyde – Primarily available as a water-based solution called formalin, which contains 37% formaldehyde by weight – is used as a high-level disinfectant and sterilant. Formaldehyde exerts its bactericidal, tuberculocidal, fungicidal, virucidal and sporicidal effects in the aqueous state, as well as in combination with low-temperature steam. This extremely reactive chemical’s mechanism of action is attributed to its interactive and cross-linking properties with protein, DNA and RNA in vitro, resulting in the disruption of DNA synthesis. It can also penetrate bacterial spores.
Formaldehyde has been traditionally used to sterilize equipment such as surgical instruments and haemodialysers in combination with alcohols. Paraformaldehyde, a solid polymer of formaldehyde, is used in combination with low-temperature steam for the disinfection of heat-sensitive medical equipment. Exposure hazards and the potential carcinogenic effects of formaldehyde have limited its healthcare uses recently, while next-generation aldehydes such as glutaraldehyde and o-phthalaldehyde, with better sporicidal activity, faster onset of action and lesser exposure effects, have replaced formaldehyde in the disinfection manuals of most healthcare establishments.
Glutaraldehyde
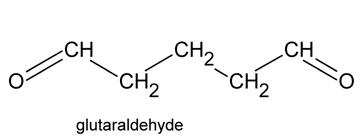

Glutaraldehyde is a saturated dialdehyde widely used as a potent sterilant and high-level disinfectant. Its broad spectrum, covering bactericidal, sporicidal, fungicidal and virucidal activity, makes it an ideal chemical for the low-temperature disinfection and sterilisation of critical and semi-critical equipment such as endoscopes, dialysers and surgical tools. Aqueous solutions of glutaraldehyde when activated (rendered alkaline at 7.5- 8.5 PH) become sporicidal, while its microbicidal action is attributed primarily to its strong association with the outer layers of bacterial cells and the alkylation of sulphydryl, hydroxyl, carboxyl and amino groups, which alter RNA, DNA and protein synthesis within microorganisms.
Glutaraldehyde is a saturated dialdehyde that has gained wide acceptance as a high-level disinfectant and chemical sterilant. Aqueous solutions of glutaraldehyde are acidic and generally in this state are not sporicidal. Only when the solution is “activated” (made alkaline) by the use of alkalinising agents to PH 7.5–8.5 does the solution become sporicidal. Once activated, these solutions have a shelf-life of minimally 14 days because of the polymerization of the glutaraldehyde molecules at alkaline PH levels. This polymerization blocks the active sites (aldehyde groups) of the glutaraldehyde molecules that are responsible for its biocidal activity.
Glutaraldehyde is also mycobactericidal, and glutaraldehyde formulations with phenols and alcohols have better usage life while maintaining excellent microbicidal activity. Glutaraldehyde exposure should be monitored to ensure a safe working environment. Acute or chronic exposure, resulting in skin irritation, mucous membrane irritation and multiple pulmonary symptoms such as occupational asthma and allergic rhinitis have been reported in healthcare workers. Due to its exposure hazards, US healthcare associations advocate the use of glutaraldehyde alternatives such as o-phthalaldehyde, hydrogen peroxide and peracetic acid.
Ortho-phthalaldehyde
Since its introduction in 1999, ortho-phthalaldehyde (OPA) has been accepted as a better, safer alternative to glutaraldehyde in most US healthcare facilities. OPA by Advanced Sterilization Products was cleared by the US FDA as a high-level disinfectant and emerged as a suitable replacement of glutaraldehyde for the disinfection of endoscopes. OPA has excellent microbiocidal activity and superior mycobactericidal activity compared with glutaraldehyde and has potent bactericidal and sporicidal activity. Like glutaraldehyde, it interacts with amino acids, proteins and microorganisms.
OPA has many advantages over glutaraldehyde, such as improved stability at varying PH ranges, lower inhalation exposure risk and a wider range of material compatibility, although it costs almost three times more; but, considering the cost of the sophisticated ventilation systems needed to minimize the respiratory hazards of using glutaraldehyde, OPA is more economical. Ortho-phthalaldehyde is a high-level disinfectant that received FDA clearance in October 1999. It contains 0.55% 1,2-benzenedicarboxaldehyde (OPA). OPA solution is a clear, pale-blue liquid with a PH of 7.5.
Hydrogen peroxide


Disinfectant solutions containing 7.5% hydrogen peroxide have been approved by the US FDA for sterilisation and high-level disinfection in healthcare settings. “Disinfectant solutions containing 7.5% hydrogen peroxide have been approved by the US FDA for sterilisation.” A 0.5% accelerated hydrogen peroxide demonstrated bactericidal and virucidal activity in 1 minute and mycobactericidal and fungicidal activity in 5 minutes. Its good, broad-spectrum bactericidal, virucidal, sporicidal and fungicidal properties, combined with its excellent stability and environmentally friendly characteristics, have made hydrogen peroxide the disinfectant of choice for semi-critical and non-critical equipment while being an ideal surface disinfectant.
Despite not being listed as equipment-compatible by all endoscope manufacturers, hydrogen peroxide-based disinfectant solutions are still considered ideal alternatives for other toxic sterilants, such as ethylene oxide and aldehyde disinfectants. Hydrogen peroxide produces destructive hydroxyl-free radicals that act on membrane lipids, DNA and other essential cell components.
Peracetic acid
Peracetic, or peroxyacetic, acid is characterized by rapid action against all microorganisms. Special advantages of peracetic acid are that it lacks harmful decomposition products (i.e., acetic acid, water, oxygen, hydrogen peroxide), enhances the removal of organic material, and leaves no residue. It remains effective in the presence of organic matter and is sporicidal even at low temperatures. Peracetic acid can corrode copper, brass, bronze, plain steel, and galvanized iron but these effects can be reduced by additives and PH modifications. It is considered unstable, particularly when diluted; for example, a 1% solution loses half its strength through hydrolysis in 6 days, whereas 40% peracetic acid loses 1%–2% of its active ingredients per month.
Another emerging alternative to ethylene oxide and aldehyde sterilants is peracetic acid. Peracetic acid-based solutions are considered to be a more potent disinfectant than hydrogen peroxide; are sporicidal, bactericidal, virucidal and fungicidal at low concentrations, and are environment friendly. They have replaced traditional disinfectants for medical devices, endoscopes and haemodialysers. It also acts as an environmental surface sterilant, and behaves similarly to other oxidising agents, disrupting cell wall permeability and oxidising sulphhydryl and sulphur bonds in proteins, enzymes and other metabolites.
Hydrogen peroxide and Peracetic acid combination


Two chemical sterilants are available that contain peracetic acid plus hydrogen peroxide (i.e., 0.08% peracetic acid plus 1.0% hydrogen peroxide and 0.23% peracetic acid plus 7.35% hydrogen peroxide. Peracetic acid, when combined with hydrogen peroxide, was found to be more effective, typically against glutaraldehyde-resistant mycobacteria. Combination of products cleared by the FDA. The combination of peracetic acid and hydrogen peroxide has been used for disinfecting haemodialysers, but the mix was found to be incompatible with flexible gastrointestinal endoscopes as claimed by leading US manufacturers.
Intermediate-level disinfectants
Sodium hypochlorite
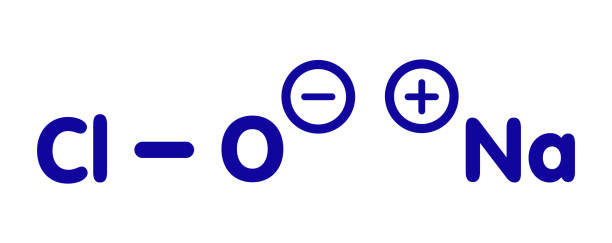
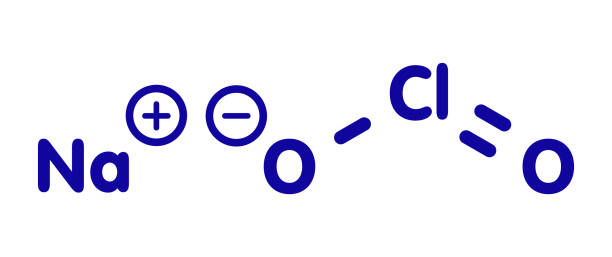



Chlorine-releasing agents (CRAs), the most popular sodium hypochlorite solution, are widely used for the disinfection of hard surfaces and blood spillages containing the human immunodeficiency virus or hepatitis B virus. Recently, sodium hypochlorite was designated as the best defence against hospital-acquired and community-acquired Clostridium difficile infections. With a broad spectrum of antimicrobial activity, sodium hypochlorite is inexpensive with low toxicity.
CRAs are highly active oxidising agents and destroy the cellular activity of proteins. Hypochlorous acid, the active moiety, has been shown to have harmful effects on bacterial DNA through the formation of chlorinated derivatives of nucleotide bases. High concentrations of sodium hypochlorite display significant levels of sporicidal and virucidal activity. Sodium hydroxide (7681-52-9)- 15-30%. Sodium hypochlorite (1310-73-2)-3-5%. The most prevalent chlorine products in the United States are aqueous solutions of 5.25%–6.15% sodium hypochlorite (see glossary), usually called household bleach.
They have a broad spectrum of antimicrobial activity, do not leave toxic residues, are unaffected by water hardness, are inexpensive and fast-acting, remove dried or fixed organisms and biofilms from surfaces and have a low incidence of serious toxicity. After reviewing environmental fate and ecologic data, EPA has determined the currently registered uses of hypochlorites will not result in unreasonable adverse effects on the environment.
Iodophors
Iodophors, complexes of iodine and a solubilising agent or carrier, are used as antiseptics and surface disinfectants. Iodine has bactericidal, fungicidal, tuberculocidal, virucidal and sporicidal properties, while its antiseptic properties are well known. Iodophors, such as povidone-iodine and poloxamer-iodine, are much more stable, have fewer irritant characteristics and exert better microbicidal action than aqueous iodine solutions. The FDA has not cleared any liquid chemical sterilant or high-level disinfectant with iodophors as the main active ingredient, but iodophors are still used in healthcare settings for disinfecting blood culture bottles and medical equipment such as thermometers.
Low-level disinfectants
Phenols

“Phenolic disinfectants disrupt the cell membrane of microorganisms.” Since Dr Joseph Lister’s use of phenols for his pioneering work on antiseptic surgery, phenolic disinfectants have been used as low and intermediate-level disinfectants. Phenolic disinfectants are effective bactericides, fungicides, tuberculocides and virucides, but are ineffective against spore-forming bacteria such as Clostridium difficile.
EPA-registered phenolic disinfectants are used to disinfect surface areas and non-critical medical devices. Phenolics are not FDA-cleared as high-level disinfectants for use with semi-critical items. Phenolic disinfectants disrupt the cell membrane of microorganisms, and two phenol derivatives used commonly in hospital disinfectants are orthophenylphenol and ortho-benzyl-parachlorophenol.
Quaternary ammonium compounds
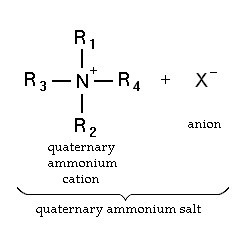
Quaternary ammonium compounds (QACs) are used for a variety of clinical purposes such as preoperative disinfection, disinfection of non-critical instruments, and hard-surface cleaning and deodorisation. QACs possess bactericidal, fungicidal and virucidal properties; however, they only display mycobacteria static and spore static activity. Quaternary ammonium disinfectants contain NH4+, the strong positive charge that results in better contact with negatively charged surfaces, making them good cleaning agents.
EPA-registered QACs are appropriate for use in disinfecting medical equipment that contacts unbroken skin, such as blood pressure cuffs. US hospitals also use quaternary ammonium-based disinfectants for the terminal disinfection of hospital rooms. Chemically, the quaternary is organically substituted ammonium compounds in which the nitrogen atom has a valence of 5, four of the substituent radicals (R1-R4) are alkyl or heterocyclic radicals of a given size or chain length, and the fifth (X‑) is a halide, sulfate, or similar radical. Each compound exhibits its own antimicrobial characteristics, hence the search for one compound with outstanding antimicrobial properties. Some of the chemical names of quaternary ammonium compounds used in healthcare are alkyl dimethyl benzyl ammonium chloride, alkyl didecyl dimethyl ammonium chloride, and dialkyl dimethyl ammonium chloride. The newer quaternary ammonium compounds (i.e., fourth-generation), referred to as twin-chain or dialkyl quaternary (e.g.) didecyl dimethyl ammonium bromide and dioctyl dimethyl ammonium bromide purportedly remain active in hard water and are tolerant of anionic residues. A few case reports have documented occupational asthma as a result of exposure to benzalkonium chloride.
Table:1 Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants
Chemical Characteristics
| Hydrogen Peroxide (7.5%) | Peracetic Acid (0.2%) | Glutaraldehyde (≥2.0%) | OPA (0.55%) | Hydrogen Peroxide / Peracetic Acid (7.35%/0.23%) |
| High-level disinfectant claim | 30 minutes @ 20°C | Not Applicable | 20-90 minutes @ 20o-25°C | 12 minutes @ 20°C, 5 minutes @ 25°C in AER | 15 minutes @ 20°C |
| Sterilization Claim | 6 hours @ 20°C | 12 minutes @ 50-56°C | 10 hours @ 20o-25°C | None | 3 h @ 20°C |
| Activation | No | No | Yes (alkaline glutaraldehyde) | No | No |
| Reuse life (number of days a product can be reused as determined by re-use protocol) | 21 days | Single-use | 14-30 days | 14 days | 14 days |
| Shelf-life stability (the time a product can remain in storage (unused)) | 2 years | 6 months | 2 years | 2 years | 2 years |
| Disposal Restrictions | None | None | Local (no U.S. EPA regulations exist but some states and local authorities have disposal restrictions) | Local (no U.S. EPA regulations exist but some states and local authorities have disposal restrictions) | None |
| Materials Compatibility | Good | Good | Excellent | Excellent | No data |
| Monitor MEC of solution | Yes (6%) | No | Yes (1.5% or higher) | Yes (0.3% OPA) | No |
| Safety | Serious eye irritant (safety glasses) | Serious eye and skin irritant (conc. Soln.) 5 | Respiratory irritant | Eye irritant, stains skin | Eye irritant |
| Processing | Manual or automated | Automated | Manual or automated | Manual or automated | Manual |
| Organic material resistance | Yes | Yes | Yes | Yes | Yes |
| OSHA exposure limit | 1 ppm TWA | None | None (The ceiling limit recommended by the American Conference of Governmental Industrial Hygienists is 0.05 ppm.) | None | Hydrogen Peroxide -1 ppm (time-weighted average for a conventional 8-hour workday) |
| Cost profile (per cycle)1 | + (manual) ++ (automated) | +++++ (automated) | + (manual) ++ (automated) | ++ (manual) | ++ (manual) |
Abbreviations and Footnotes: OPA ortho-phthalaldehyde (FDA cleared as a high-level disinfectant, included for comparison to other chemical agents used for high-level disinfection)
AER Automated Endoscope Reprocessor. MEC minimum effective concentration is the lowest concentration of active ingredients at which the product is still effective.
1 per cycle cost profile considers the cost of the processing solution (suggested list price to healthcare facilities in August 2001) and assumes maximum use life (e.g., 21 days for hydrogen peroxide, 14 days for glutaraldehyde), 5 reprocessing cycles per day, 1-gallon basin for manual processing, and 4-gallon tank for automated processing.
+ = least expensive; +++++ = most expensive
Table 2: Summary of advantages and disadvantages of chemical agents used as chemical sterilants1 or as high-level disinfectants
Sterilization Method
| Advantages | Disadvantages |
| Peracetic Acid/Hydrogen Peroxide |
|
|
| Glutaraldehyde |
|
|
| Hydrogen Peroxide |
|
|
| Ortho-phthalaldehyde |
|
|
| Peracetic Acid |
|
|
Modified from Rutala
1. All products effective in presence of organic soil, relatively easy to use, and have a broad spectrum of antimicrobial activity (bacteria, fungi, viruses, bacterial spores, and mycobacteria). The above characteristics are documented in the literature; contact the manufacturer of the instrument and sterilant for additional information. All products listed above are FDA-cleared as chemical sterilants except OPA, which is an FDA-cleared high-level disinfectant.