Wastewater treatment is a process used to remove contaminants from wastewater and convert it into an effluent that can be returned to the water cycle. Once returned to the water cycle, the effluent creates an acceptable impact on the environment or is reused for various purposes (called water reclamation).
The treatment process takes place in a wastewater treatment plant. There are several kinds of wastewater that are treated at the appropriate type of wastewater treatment plant. For domestic wastewater (also called municipal wastewater or sewage), the treatment plant is called a sewage treatment plant. For industrial wastewater, treatment either takes place in a separate industrial wastewater treatment plant, or in a sewage treatment plant (usually after some form of pre-treatment). Further types of wastewater treatment plants include agricultural wastewater treatment plants and leachate treatment plants.
Processes commonly used include phase separation (such as sedimentation), biological and chemical processes (such as oxidation) or polishing. The main by-product from wastewater treatment plants is a type of sludge that is usually treated in the same or another wastewater treatment plant. Biogas can be another by-product if anaerobic treatment processes are used.
Some wastewater may be highly treated and reused as reclaimed water. The main purpose of wastewater treatment is for the treated wastewater to be able to be disposed of or reused safely. However, before it is treated, the options for disposal or reuse must be considered so the correct treatment process is used on the wastewater.
Types of treatment plants:
Wastewater treatment plants may be distinguished by the type of wastewater to be treated. There are numerous processes that can be used to treat wastewater depending on the type and extent of the contamination. The treatment steps include physical, chemical and biological treatment processes.
Types of wastewater treatment plants include:
- Sewage treatment plants
- Industrial wastewater treatment plants
- Agricultural wastewater treatment plats
- Leachate treatment plants
Sewage treatment plants
Sewage treatment (or domestic wastewater treatment, municipal wastewater treatment) is a type of wastewater treatment that aims to remove contaminants from sewage. Sewage contains wastewater from households and businesses and possibly pre-treated industrial wastewater. Physical, chemical, and biological processes are used to remove contaminants and produce treated wastewater (or treated effluent) that is safe enough for release into the environment. A by-product of sewage treatment is a semi-solid waste or slurry, called sewage sludge. The sludge has to undergo further treatment before being suitable for disposal or application to land. The term “sewage treatment plant” is often used interchangeably with the term “wastewater treatment plant”.
For most cities, the sewer system will also carry a proportion of industrial effluent to the sewage treatment plant that has usually received pretreatment at the factories to reduce the pollutant load. If the sewer system is a combined sewer, then it will also carry urban runoff (stormwater) to the sewage treatment plant. Sewage is conveyed in sewerage which comprises the drains, pipework and pumps to convey the sewage to the treatment works inlet. The treatment of municipal wastewater is part of the field of sanitation. Sanitation also includes the management of human waste and solid waste as well as stormwater (drainage) management.
At the global level, an estimated 52% of municipal wastewater is treated. However, wastewater treatment rates are highly unequal for different countries around the world. For example, while high-income countries treat approximately 74% of their municipal wastewater, developing countries treat an average of just 4.2%. Wastewater that is discharged untreated into the environment can cause water pollution.
In developing countries and in rural areas with low population densities, sewage is often treated by various on-site sanitation systems and not conveyed in sewers. These systems include septic tanks connected to drain fields, on-site sewage systems (OSS), vermin filter systems and many more. A typical sewage treatment plant in a high-income country may include primary treatment to remove solid material, secondary treatment to digest dissolved and suspended organic material as well as the nutrients nitrogen and phosphorus, and – sometimes but not always – disinfection to kill pathogenic bacteria. Sewage can also be treated by processes using “Natural-based solutions”.
Industrial wastewater treatment plants
Industrial wastewater treatment describes the processes used for treating wastewater that is produced by industries as an undesirable by-product. After treatment, the treated industrial wastewater (or effluent) may be reused or released to a sanitary sewer or to surface water in the environment. Most industrial processes, such as petroleum refineries, chemical and petrochemical plants have onsite facilities to treat their wastewaters so that the pollutant concentrations in the treated wastewater comply with the regulations regarding disposal of wastewaters into sewers or into rivers, lakes or oceans. Industrial wastewater treatment plants are required where municipal sewage treatment plants are unavailable, do not have sufficient capacity or cannot adequately treat specific industrial wastewaters.
Most industries produce some wastewater. Recent trends have been to minimize such production or to recycle treated wastewater within the production process. Sources of industrial wastewater include battery manufacturing, electric power plants, food industry, iron and steel industry, mines and quarries, nuclear industry, oil and gas extraction, organic chemicals manufacturing, petroleum refining and petrochemicals, pulp and paper industry, smelters, textile mills, industrial oil contamination, water treatment, wood preserving. Treatment processes include brine treatment, solids removal (e.g., chemical precipitation, filtration), oils and grease removal, removal of biodegradable organics, removal of other organics, removal of acids and alkalis, removal of toxic materials.
Agricultural wastewater treatment plants
Agricultural wastewater treatment is a farm management agenda for controlling pollution from confined animal operations and from surface runoff that may be contaminated by chemicals in fertilizer, pesticides, animal slurry, crop residues or irrigation water. Agricultural wastewater treatment is required for continuous confined animal operations like milk and egg production may be performed in plants using mechanized treatment units similar to those used for industrial wastewater; but where land is available for ponds, settling basins and facultative lagoons may have lower operational costs for seasonal use conditions from breeding or harvest cycles.
Many farms generate nonpoint source pollution from surface runoff which is not controlled through a treatment plant. Farmers can install erosion controls and implement nutrient management plans to control runoff pollution. Nonpoint source pollution includes sediment runoff, nutrient runoff and pesticides. Point source pollution includes animal wastes, silage liquor, milking parlour (dairy farming) wastes, slaughtering waste, vegetable washing water and firewater.
Leachate treatment plants
A leachate is any liquid that, in the course of passing through matter, extracts soluble or suspended solids, or any other component of the material through which it has passed.
Leachate is a widely used term in the environmental sciences where it has the specific meaning of a liquid that has dissolved or entrained environmentally harmful substances that may then enter the environment. It is most commonly used in the context of land-filling of putrescible or industrial waste. In the narrow environmental context leachate is therefore any liquid material that drains from land or stockpiled material and contains significantly elevated concentrations of undesirable material derived from the material that it has passed through.
Why Treat Wastewater?
It’s a matter of caring for our environment and for our own health. There are a lot of good reasons why keeping our water clean is an important priority:
FISHERIES: Clean water is critical to plants and animals that live in water. This is important to the fishing industry, sport fishing enthusiasts, and future generations.
WILDLIFE HABITATS: Our rivers and ocean waters teem with life that depends on shorelines, beaches and marshes. They are critical habitats for hundreds of species of fish and other aquatic life. Migratory waterbirds use the areas for resting and feeding.
RECREATION AND QUALITY OF LIFE: Water is a great playground for us all. The scenic and recreational values of our waters are reasoning many people choose to live where they do. Visitors are drawn to water activities such as swimming, fishing, boating and picnicking.
HEALTH CONCERNS: If it is not properly cleaned, water can carry disease. Since we live, work and play so close to water, harmful bacteria have to be removed to make water safe.
Effects of wastewater pollutants
If wastewater is not properly treated, then the environment and human health can be negatively impacted. These impacts can include harm to fish and wildlife populations, oxygen depletion, beach closures and other restrictions on recreational water use, restrictions on fish and shellfish harvesting and contamination of drinking water.
Environment Agency provides some examples of pollutants that can be found in wastewater and the potentially harmful effects these substances can have on ecosystems and human health:
- Decaying organic matter and debris can use up the dissolved Oxygen in a lake so fish and another aquatic biota cannot survive;
- Excessive nutrients, such as Phosphorus and nitrogen (including ammonia), can cause eutrophication, or over-fertilization of receiving waters, which can be toxic to aquatic organisms, promote excessive plant growth, reduce available oxygen, harm spawning grounds, alter habitat and lead to a decline in certain species;
- Chlorine compounds and inorganic chloramines can be toxic to aquatic invertebrates, algae and fish;
- Bacteria, viruses and disease-causing pathogens can pollute beaches and contaminate shellfish populations, leading to restrictions on human recreation, drinking water consumption and shellfish consumption;
- Metals, such as mercury, lead, cadmium, chromium and arsenic can have acute and chronic toxic effects on species.
- Other substances such as some Pharmaceuticals and personal care products, primarily entering the environment in wastewater effluents, may also pose threats to human health, aquatic life and wildlife.
Wastewater treatment
The major aim of wastewater treatment is to remove as much of the suspended solids as possible before the remaining water, called effluent, is discharged back to the environment. As solid material decays, it uses up oxygen, which is needed by the plants and animals living in the water.
“Primary treatment” removes about 60 per cent of suspended solids from wastewater. This treatment also involves aerating (stirring up) the wastewater, to put oxygen back in. Secondary treatment removes more than 90 per cent of suspended solids.
What is water pollution?
Water pollution is the contamination of water bodies (lakes, rivers, oceans, aquifers, and groundwater), very often by human activities.
Water pollution occurs when pollutants (particles, chemicals, or substances that make water contaminated) are discharged directly or indirectly into water bodies without enough treatment to get rid of harmful compounds. Pollutants get into water mainly by human causes or human factors.
Water pollution is the second most imperative environmental concern along with air pollution. Any change or modification in the physical, chemical, and biological properties of water that will have a detrimental consequence on living things, is water pollution.
The water pollution problem
Water covers over 70% of the Earth’s surface. It is an important resource for people and the environment. Water is crucial to human health. The WHO advises that at least 7.5 litres per day per person are necessary to meet “the requirements of most people under most conditions” and at least another 20 litres per day to cover basic hygienic needs.
WHAT IS WASTEWATER?
Wastewater is water that has been used and must be treated before it is released into another body of water so that it does not cause further pollution of water sources. Wastewater comes from a variety of sources. Everything that you flush down your toilet or rinse down the drain is wastewater. Rainwater and runoff, along with various pollutants, go down street gutters and eventually end up at a wastewater treatment facility. Wastewater can also come from agricultural and industrial sources. Some wastewater can be more difficult to treat than others; for example, industrial wastewater can be difficult to treat, whereas domestic wastewater is relatively easy to treat (though it is increasingly difficult to treat domestic waste, due to increased amounts of pharmaceuticals and personal care products that are found in domestic wastewater.)
Breaking Down the Chemicals Used in Wastewater Treatment
Wastewater treatment is a complex process that requires a variety of chemicals to modify the pH of the water and prevent the release of pollutants, such as heavy metals, volatile and semi-volatile organic compounds and pesticides into the surrounding environment. Chemical treatment of wastewater is used by industries, such as pharmaceutical, energy production, and paper production. Below we discuss the primary chemicals used in wastewater treatment and their common applications.
Types of Wastewater Treatment Chemicals:
To meet both governmental regulations and industry standards for chemical effluents and pollutants, numerous wastewater chemical solutions have been developed. Depending on the application, one or more chemical treatments may be necessary to produce up-to-code wastewater.
Coagulants
Coagulants are aluminium or iron-based chemicals that change the magnetic charge of particles in the water, causing them to attract instead of repelling each other. The waste coalesces into larger bodies that can be skimmed or filtered out. Materials often removed from wastewater with coagulants include arsenic, pathogens, organic matter, fluoride, and chemical phosphorus.
Odor Control
Although odour control is a secondary consideration when compared with the detection and removal of highly toxic chemicals, a range of effective methods are available to reduce unwanted smells and effusions, including biofiltration, solids scavenging, oxidation, iron salts, carbon absorption, and liquid phase technology.
The most toxic contaminants of industrial waste often smell innocuous; however, these dangerous contaminants require chemical treatment even before biological water treatment can be considered. Hexavalent chromium, for example, can leach into groundwater undetected, ultimately causing birth defects, cancer, and other chronic and terminal illnesses. The notion of highly toxic wastes that are colourless, odourless, and otherwise invisible was brought to mainstream consciousness, and industry standards have since been adjusted accordingly.
Flocculants
Flocculants are divided into three categories: natural, chemical, and grafted. Coupled with water agitation processes, flocculants encourage waste particles to adhere to one another in clumps, or flocs, through charge bridging, electrostatic patching, and magnetic neutralization.
Defoamers
Defoamers, as the name implies, are used to control and reduce the level of trapped air and foam created during wastewater treatment processes. This helps to decrease the potential for dangerous overflow.
Organic Polymers
Organic polymers are natural flocculants that use an ionized polymer to attract particles into flocs for easier filtration. They are often used in conjunction with inorganic iron or aluminium-based coagulants.
Reducing Agents
Reducing agents, also known as oxidizing agents, include sodium bisulfite, sodium hydrosulfite, and ferrous sulfate. They are typically used to remove harmful substances, such as ozone, hydrogen peroxide, chlorine, and biological contaminants, from wastewater. Coupled with aeration, these chemicals bond to suspended compounds to reduce them to component parts that can settle out of the water and be flocculated and filtered.
Sludge Conditioners
Chemical sediment and waste biomass can be difficult to filter and may require additional conditioning through heat or chemical treatments to thicken the material, reduce the odour, and decrease the sludge volume. This process makes it easier for the sludge to be removed and safely disposed of.
Cleaners and Degreasers
Cleaners and degreasers, such as clog busters and alkaline drain openers, are formulated to dissolve grease and oil. They use solvent chemicals to break down the grease to make it easier to remove.
Membrane Cleaners and Antiscalants
Membrane cleaners and antiscalants are injected into wastewater prior to treatment, to prevent dissolved mineral salts from coalescing on filtration membranes, thereby ensuring adequate fluid flow. Common membrane cleaners and antiscalants used in water treatment applications include:
- Chlorine Dioxide
- Muriatic Acid
- Soda Ash
- Algicide
- Chlorine
- Sodium Bicarbonate
Biocides and Bio-Dispersants
Biocides and bio-dispersants reduce microorganisms in wastewater, enhancing the operational efficiency of wastewater treatment operations. Biocides include cleaners, scale removers, dispersants, penetrants, and disinfectants.
Heavy Metal Precipitants
Heavy metals in wastewater are often ionically bonded with chelating agents or other bonding chemicals that prevent them from becoming soluble. Heavy metal precipitants dissolve those bonds allowing dangerous heavy metals to precipitate out of the wastewater.
pH Control
pH control adds acidic or basic chemicals to the wastewater, thereby allowing hydroxide ions to bond with heavy metals and precipitate out of the solution. In addition, greater acidity will kill bacteria and organic compounds by breaking them down at a cellular level.
Wastewater treatment in developing countries is a major concern and the solution has become challenging for various unfavourable conditions. Inadequate education and low economic perspective are causing difficulties in implementing advanced treatment methods. Developing countries are also facing several water-related problems both in the urban and rural regions. Waterborne diseases are a common phenomenon among the villagers as well as for urban inhabitants.
Insufficiency in a wastewater treatment facility is making effluent water harmful to the environment. Most of the untreated effluent is discharged to the nearest water bodies. Groundwater depletion is increasing with time. The country requires some urgent solutions to eradicate problems regarding wastewater.
High population density and economic adversity are making difficulties in implementing the solution. Moreover, political issues and social restrictions have a huge impact on decision making. Natural calamities such as flooding affect the country very frequently. This also makes an adverse effect on the water sector. Decentralized wastewater treatment can make a remarkable change in the wastewater issues in Bangladesh.
The reuse of effluent water in the agricultural fields is another perspective of the decentralized system. Treated effluent could be used further in irrigation. This initiative can positively reduce pressure on groundwater as well as energy consumption.
Finally, this is very alarming for the future generation of Bangladesh if we do not start properly the wastewater treatment management by using eco-friendly, biodegradable chemical agents with no residual effect on the environment.
Four common ways to treat wastewater include physical water treatment, biological water treatment, chemical treatment, and sludge treatment. Let us learn about these processes in detail. In this stage, physical methods are used for cleaning the wastewater.
Best Method for Wastewater Treatment
Wastewater is generally defined as water that has already been used in some capacity. Water from homes, businesses or industries is considered wastewater. Melted snow and runoff water from outdoor activities is also wastewater. There are several important reasons why you need to pay careful attention to how wastewater is processed and the different methods by which to treat it.
WHY TREAT WASTEWATER?
Wastewater can contain chemical, biological or physical pollutants. This can make it unsafe for human use. It can potentially cause severe illness if untreated wastewater gets into the public drinking supply. Most wastewater is usually released back into the environment after treatment.
WHAT TREATMENT PROCESSES ARE USED?
There are several steps you would normally take when treating wastewater in a municipal facility. According to Environmental Protection Agency, wastewater goes through five distinct processes that include preliminary, primary and secondary treatments, as well as disinfection and sludge treatment. Most treatment facilities employ similar steps or combine steps when treating wastewater.
- PRELIMINARY/PRIMARY
Preliminary treatment normally includes screening the water to remove large objects and debris. Wastewater pretreatment can include everything from twigs and rocks to bottles and diapers. For industrial users, the nation pollutant discharge elimination system sets wastewater pretreatment standards that are stricter.
- SECONDARY
This is where your treatment options begin to diverge. Coagulation, along with flocculation, is a method that requires a combination of chemicals. These processes cause particles to stick together so at a later point they can be more easily filtered out. Aluminium sulfate is a chemical often used in this process. After these insoluble fragments settle at the bottom through sedimentation, the purified water is filtered out. Filtration involves using a variety of filters to catch particles as the water flows through.
- DISINFECTION
This is sometimes referred to as the tertiary treatment phase. Chlorine and chloramines are chemicals often used during the water treatment disinfection process. UV radiation is also sometimes used to disinfect water.
- SLUDGE TREATMENT
The final stage of treating water will often include removing a sludge that is sometimes referred to as biosolids. According to Water Use it Wisely, the byproduct of the sludge dewatering system is sometimes used for agricultural purposes.
WHAT TREATMENT METHODS ARE BEST?
The previous section details the processes involved in treating wastewater. Biotech articles state that the specific methods used generally fall into three categories.
BIOLOGICAL
Biological methods are normally put in place when the water will be used for drinking purposes. Aerobic treatment and fermentation are both biological methods.
PHYSICAL
Physical methods include sedimentation, aeration and filtration. Sand filters are sometimes used in the oil-water separation process to remove oil and grease particles.
CHEMICAL
Chlorine is the chemical most often used in treating sewage and other types of wastewater. The process is called chlorination. This is the most effective means of destroying a variety of viruses and bacteria. A method known as neutralization is effective when treating industrial wastewater. Lime is sometimes used when treating acidic water.
What treatment solutions you’ll need will likely be determined by the type of wastewater, what contaminants are in the water and what the water will be used for after it’s treated. The best methods for treating wastewater should always coincide with regulations required in the state and locality where your facility is located. The methods used should also be as environmentally safe as possible.
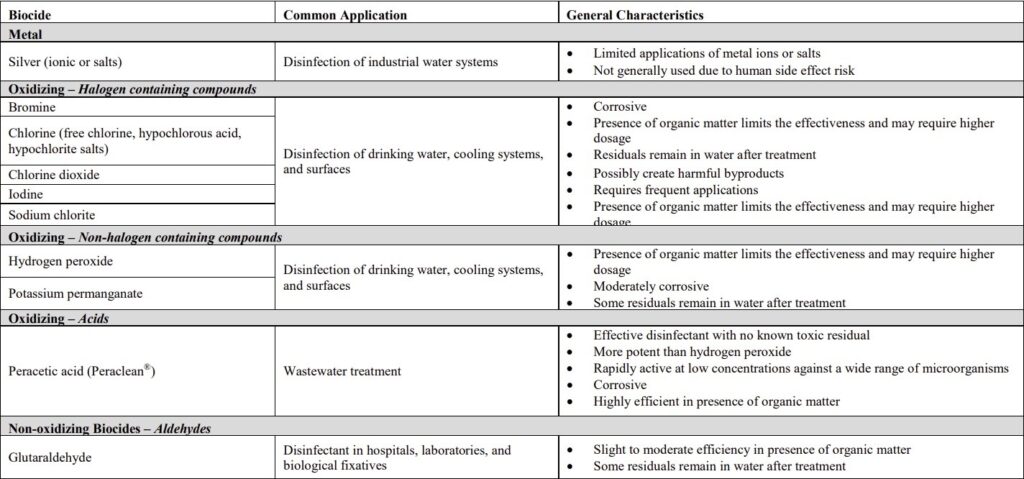
Biocide chemicals used in Wastewater treatment plant
There are two basic types of biocides: oxidising and non-oxidising. Oxidising biocides include; chlorine, chlorine dioxide, hydrogen peroxide, bromine and ozone. Non-oxidising biocides include quaternary ammonium compounds, Bronopol, THPS, DBNPA and Glutaraldehyde.
When selecting a biocide for use in a system whether as a biocidal flush biocide, maintenance dose biocide or a shock dose biocide a number of factors need to be considered including contact time, concentration, temperature, pH, compatibility, efficacy against microbes present in the system and cost-effectiveness. Our technical department can advise on biocide selection.
Biocides & Disinfectants
Our high-performance biocides deliver superior performance and include both oxidising and non-oxidising biocides for complete water system control.
The control of microbiological activity is an important, often safety-critical activity in many commercial, manufacturing and industrial process applications such as Cooling Towers, Closed systems, Reverse Osmosis & Cold-Water Storage Tanks.
To ensure that such systems are safe, well maintained and operate at optimum efficiency it is essential that the correct water conditions are maintained at all times. This can be achieved using carefully selected biocides.
Our oxidising biocides include stabilized bromine and chlorine tablets, hydrogen peroxide silver, and sodium bromide-based bromine precursors.
Our non-oxidising biocides include a range of broad-spectrum biocides and micro-biocides, Glutaraldehyde, quaternary ammonium compounds (QAC), and more
Closed System Biocides
The accumulation of microbiological slimes, biofilm and general bio-fouling in closed circuit water systems can be problematic. The build-up of such unwanted foulants can reduce system efficiency, and increase operating and maintenance costs.
Closed heating & cooling systems can become a breeding ground for a range of troublesome microbes if they are untreated. These include such micro-organisms as pseudomonas, nitrifying and sulphate reducing bacteria. They can cause fouling, destroy closed system inhibitors and cause MIC (microbially induced corrosion).
A biocide is essential to prevent slime growth in chilled water loops and we would always recommend a closed-circuit biocide is incorporated in any system operating below 60°C. PuraHub’s range of tried and tested disinfectants and biocides are designed to be particularly suited to closed system treatment.
Scale & Corrosion Inhibitors
The unwanted formation of scale and detrimental impact of metallic corrosion can be significant issues that affect the operation and maintenance of closed-circuit water systems.
Closed System Inhibitors
Closed-circuit heating and cooling systems must be well maintained to operate at optimum efficiency so it is essential that the correct water conditions are maintained at all times. This can be achieved using carefully selected inhibitors designed to control corrosion, scale/fouling and maintain flow and heat transfer.
Our advanced closed-circuit inhibitors have been scientifically formulated to protect closed systems including closed chilled water, low, medium and high temperature/pressure heating systems from the effects of both metallic corrosion and fouling all within a single product. PuraHub’s scale & corrosion inhibitors deliver improved performance, helping to reduce maintenance costs, improve reliability and optimise operational efficiency
Reducing Corrosion & Scale Risk in Closed Systems
There are multiple factors that can increase or decrease the risk of scale or corrosion within a closed water system most of which are briefly detailed below, summarized:
Corrosion factors:
- Dissolved Oxygen – most important factor to govern corrosion
- Dissolved Carbon Dioxide – can cause a reduction in pH
- pH – pH below 7 may see rapid corrosion (Aluminium corrosion can occur at high pH so treatment program should be limited to pH of 8.5)
- Temperature – higher temperatures tend to increase rates of corrosion
- TDS (Total Dissolved Solids) – high conductivity aids certain corrosion processes
- Galvanic – Aluminium & Copper corrosion due to ‘electrochemical potential difference’
- Sulphates – high levels can increase corrosion, additionally, sulphate ions may be consumed by SRBs (Sulphates Reducing Bacteria) to cause pitting
- Bacteria – risk of corrosion from aerobic & anaerobic bacteria
- Chloride attack – high levels can interfere with the development of protective oxide films on surfaces
- Flow Velocity – turbulent erosion on softer metals (low risk)
- Surface Condition – dirt & contamination may encourage localized corrosion
- Stress – stress-corrosion cracking
Note on Aluminium: Closed systems containing aluminium are considered high risk due to the potential for galvanic corrosion, the decision on chemical treatment should be carefully considered as it may require specific inhibitors and a higher than typical dosage to maintain adequate protection. We recommend a Synergized Molybdate treatment for these systems.
Note on Hard Water: The permanent or temporary installation of a water softener may be required where the available water is quite hard and especially where there are lots of leaks as these can leave abrasive evaporation deposits & form scale. Please note, the make-up water should not be entirely softened as the total removal of calcium can make the water more corrosive. However, be aware an underused water softener may become a source of bacterial contamination and may not be ideally suited for applications where its sole purpose is the supply to the closed system.
The use of Scale & Corrosion inhibitors
A closed system inhibitor works by laying down a thin film over the surface of the system metal and should be chosen carefully taking into account suitability for all materials used within the system. It is important that the recommended chemical reserve is maintained in the system to cover for a minor loss of water within the system, or general degradation and absorption of chemicals within the heating system, the aim is to only require occasional chemical top-ups.
pH is generally kept within a range of 8 – 11 to promote a less corrosive environment however as noted above systems with aluminium should be kept around 8.5 as the metal is known to corrode at higher pH levels.
Biocides Considered for Potential Treatment of Ballast Water
WASTEWATER TREATMENT WITH PERACETIC ACID
WASTEWATER TREATMENT WITH PERACETIC ACID
We understand the need to disinfect wastewater with treatment chemicals that address the concerns associated with chlorination – safety, toxicity, by-product formation and cost. That’s why they’ve developed VIGOROX® WWT II wastewater disinfection technology, the environmentally responsible and economically minded alternative disinfectant to chlorine and UV disinfection. VIGOROX® WWT II, a formulation containing 15% peracetic acid and 23% hydrogen peroxide, represents an innovative solution in industrial wastewater treatment.
We have recently worked with the Standard Methods Organization for the examination of water and wastewater and other industry professionals to develop a method for the analysis of peracetic acid (PAA) residuals. This PAA-specific method will enable wastewater professionals to accurately measure residuals in a plant’s outflow to meet their National Pollutant Discharge Elimination System permit. Method 4500-PAA is now available for use.
WASTE WATER TREATMENT WITH HYDROGEN PEROXIDE
Nothing is more important than safe, clean water. Evonik’s portfolio of environmental treatment technologies includes safe chemistries to eliminate impurities and disinfect water. More importantly, our water treatment chemistries are backed by the robust technical expertise and years of experience of our scientists.
Hydrogen peroxide is a strong oxidizing agent that is used in pre-oxidation for algae control, precipitation of iron and manganese and for the oxidation of many kinds of dissolved substances, both organic and inorganic. Furthermore, in biologically active wastewater, hydrogen peroxide will readily decompose to water and oxygen. The release of oxygen can assist in biological oxygen demand (BOD) reduction by allowing the aerobic bacteria to function more efficiently.
This mechanism is especially important in cases where an oxygen deficiency exists due to high biological oxygen demand/chemical oxygen demand (COD) / Total organic carbon (TOC) loadings and/or insufficient aeration.
WASTEWATER TREATMENT WITH AOP
In the Advanced Oxidation Process (AOP) hydrogen peroxide is activated by additional components, such as iron salts (Fenton`s reagent), ozone or ultraviolet light. The AOP technology is one of the most commonly applied approaches for wastewater treatment. It is successfully used for the degradation of recalcitrant pollutants in various refinery effluents and wastewater especially from chemical or Pharmaceutical facilities.
The high efficiency of this process is based on the oxidation power of highly reactive hydroxyl radicals, which are formed by the activation of hydrogen peroxide.
TREATMENT OF RECYCLING WATER
Water recycling is one of the most suitable methods for saving drinking water and reducing wastewater. In many applications, it is possible to recycle the process or rinse water. Therefore, this approach has an enormously beneficial impact on the environment. On the other hand, users are faced with the challenge of reducing the increased level of contaminants in recycled water. The presence of biodegradable organic compounds can result in the proliferation of microorganisms along with associated visual and odour problems.
Good examples are car wash facilities in which the majority of the washing water is recycled.
Bad odours resulting from the metabolic processes of microorganisms can be avoided by continuously dosing small amounts of biocides, e.g., peracetic acid into the recycled water. In case of acute odour issues, it is also possible to carry out a shock dosage of peracetic acid. The application of peracetic acid permits the successful use of recycled water and therefore has a direct benefit for the environment.

PuraHub Limited marketed the following certified approved company’s products
Contact Us
Have questions or need help? Use the form to reach out and we will be in touch with you as quickly as possible.